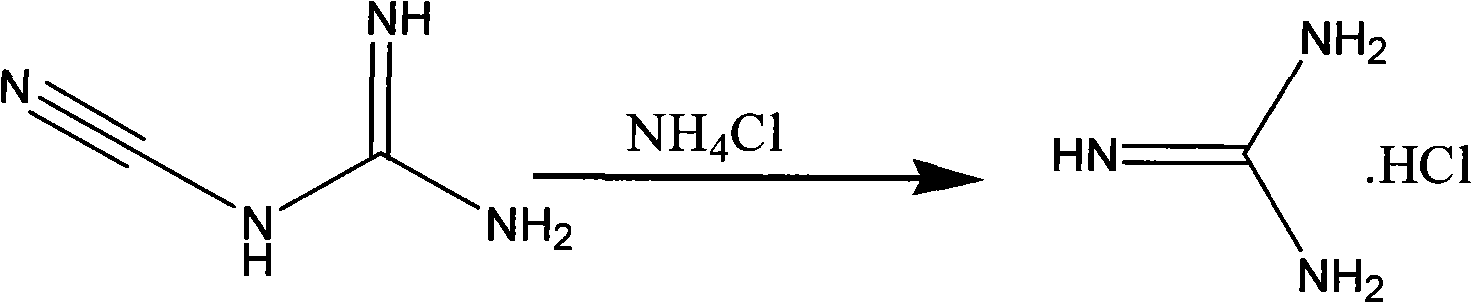
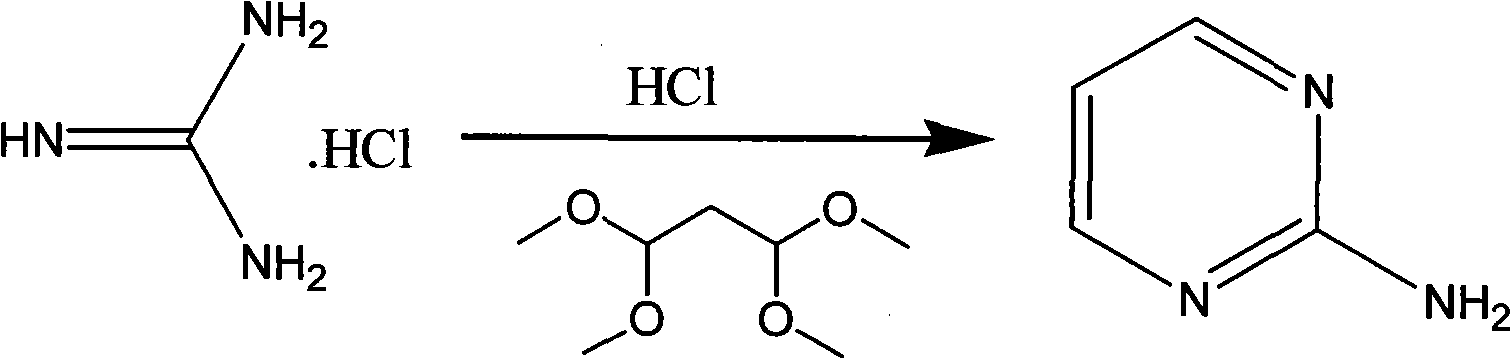
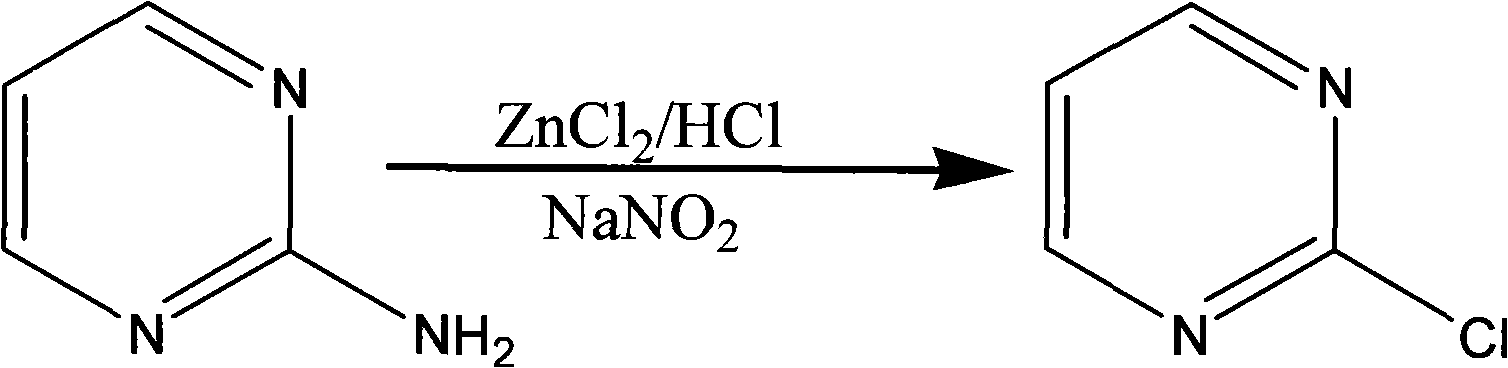Method for preparing 2-chloropyrimidine
A technology for chloropyrimidine and aminopyrimidine, applied in the field of preparing 2-chloropyrimidine, can solve the problems of high cost, low yield, high cost and the like, and achieve the effects of saving raw material cost, simple process flow and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Preparation of 2-chloropyrimidine
[0027] Add 8.41 grams of dicyandiamide and 16.05 grams of ammonium chloride into a 250ml four-necked reaction flask, then slowly raise the temperature to 150-180°C, and maintain this temperature for 8 hours. Industrial hydrochloric acid, 16 grams of 1.1.3.3-tetramethoxypropane, heat up to 70-85°C, maintain this temperature for 5 hours, keep warm, distill to dryness under reduced pressure at 70-85°C, add 35.5 grams of industrial hydrochloric acid, and then cool down To 10-20°C, maintain this temperature and add 41.1 grams of zinc chloride in batches, after adding, maintain this temperature for 0.5 hours, then cool down to -20--10°C, maintain this temperature, slowly add 58.5 grams of 41% sub- Sodium nitrate aqueous solution, add and keep warm for 0.5 hours, add 100 grams of ice water after keeping warm, then add 100ml, 100ml, 100ml, 100ml, 100ml of dichloromethane to extract five times, discard the water layer, combine the ...
Embodiment 2
[0028] Example 2: Preparation of 2-chloropyrimidine
[0029] According to Example 1, change dicyandiamide to 6 grams, and others are the same, finally obtaining 5.55 grams of 2-chloropyrimidine.
Embodiment 3
[0030] Example 3: Preparation of 2-chloropyrimidine
[0031] According to embodiment 1, change dicyandiamide into 20 grams, others are the same as above, finally obtain 7.18 grams of 2-chloropyrimidine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com