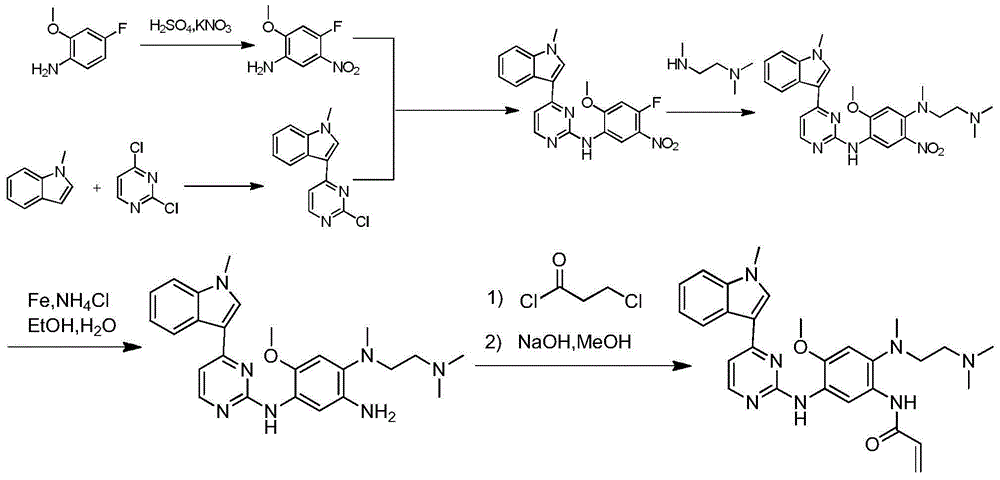
Synthetic method of anti-tumor medicine
A technology of anti-tumor drugs and synthetic methods, which is applied in the direction of anti-tumor drugs, drug combinations, chemical instruments and methods, etc., can solve the problems of AZD9291 industrial production difficulties, achieve the effects of lowering the temperature, avoiding complicated operations, and increasing the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
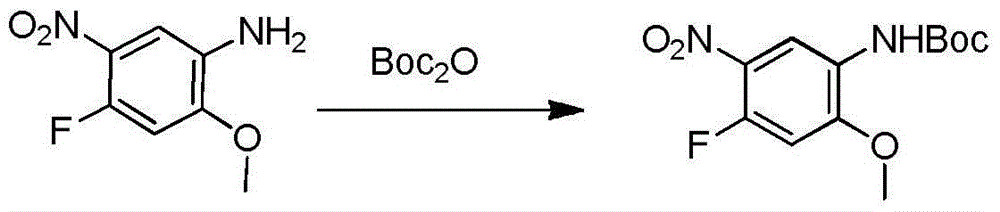
[0039] Example 1: Preparation of compound 4-fluoro-2-methoxyl-5-nitroanilinocarbamate tert-butyl
[0040] Add 500mg (2.69mmol) of 4-fluoro-2-methoxy-5-nitroaniline, 586.24mg (2.69mmol) of Boc anhydride and 10ml of acetonitrile into a single-necked bottle, raise the temperature to 55°C for 6 hours, and spin off under reduced pressure. Acetonitrile, 5ml of ethyl acetate was added to dissolve the system, and 1ml of petroleum ether was slowly added under stirring to precipitate a solid, which was filtered to obtain 639mg of a yellow solid, with a yield of 84%.
Embodiment 2
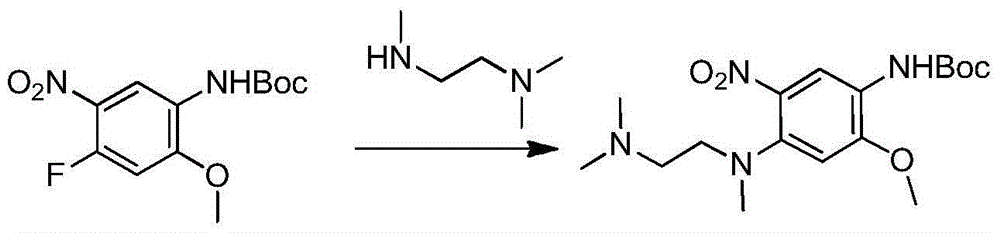
[0041] Example 2: Preparation of compound 4-(N,N,N'-trimethylethylenediamine)-2-methoxyl-5-nitroaniline formate tert-butyl
[0042] Under nitrogen / argon protection, 200mg (0.698mmol) tert-butyl 4-fluoro-2-methoxy-5-nitroanilinocarbamate, 78.47mg (0.768mmol) N,N,N'-trimethyl Add ethylenediamine, 117.27mg (0.907mmol) N,N-diisopropylethylamine and 10ml N,N-methylacetamide into a 50ml one-necked flask, raise the temperature to 45°C, stir for 2h, cool down to room temperature, add 40ml of water was extracted twice with 20ml of dichloromethane, the organic layers were combined, washed with saturated brine, dried, filtered with suction, and spin-dried with dichloromethane to obtain 252mg of a bright orange solid with a yield of 98%.
Embodiment 3
[0043] Embodiment 3: Preparation of compound 2-(N,N,N'-trimethylethylenediamino)-4-methoxy-5-carbamic acid tert-butyl aniline
[0044] Under nitrogen / argon protection, 500mg (1.3mmol) tert-butyl 4-(N,N,N'-trimethylethylenediamine)-2-methoxy-5-nitroaniline carbamate, 2.5 mg palladium carbon and 50ml methanol were added to a 100ml single-necked flask, the system was replaced with hydrogen three times, stirred and reacted at room temperature for 2h, a layer of diatomaceous earth was placed on the filter paper, the system was filtered, and the mother liquor was spin-dried to obtain a brown oily substance 422.4 mg, yield 96%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com