Synthesis method of 2-chloropyrimidine
A synthetic method, the technology of chloropyrimidine, which is applied in the field of synthesis of 2-chloropyrimidine, can solve the problems of low yield and low product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
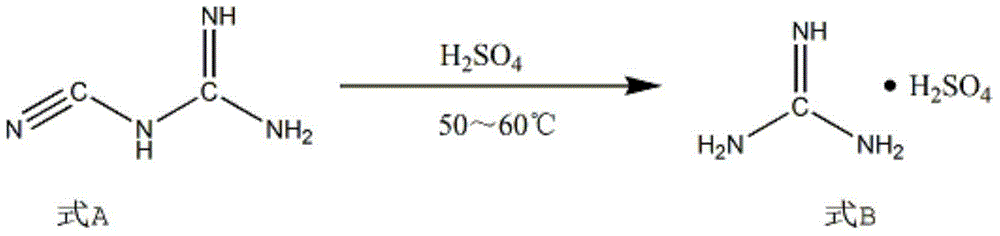
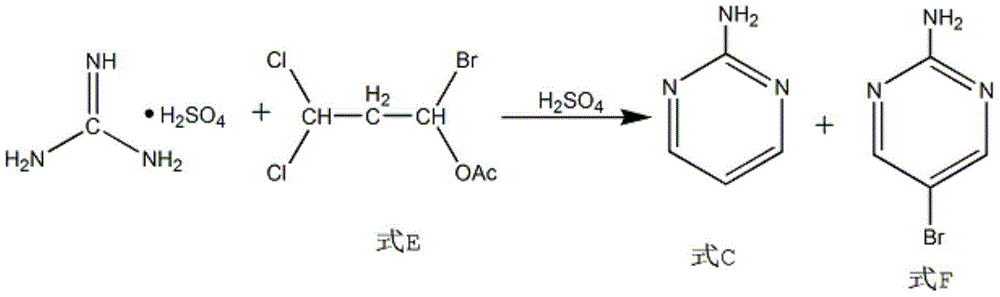
[0021] Add 1.5 mol of solute and 96 wt% sulfuric acid into a 250ml four-necked flask, adjust its temperature to 2°C, slowly add 1mol of dicyandiamide to the four-necked flask while stirring constantly, and raise the temperature to 50°C to make it Hydrolysis reaction 6h. Then cool to room temperature. Add 0.435mol 1-bromo-1-acetyl-3,3-dichloropropane into the four-neck flask, first adjust the temperature to 50°C and maintain it for 2h, then raise the temperature to 55°C and maintain it for 6h. The product is extracted with toluene extractant and cooled with ice water to obtain 2-aminopyrimidine.
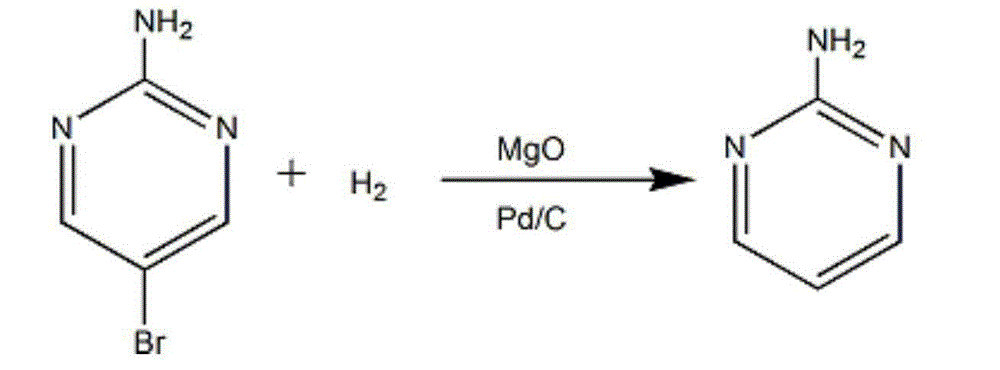
[0022] Add a solvent of ethanol and water with a volume ratio of 1:2, a palladium carbon catalyst, and magnesium oxide to the remaining cyclization reaction product after extraction, and control the quality of the palladium carbon catalyst, magnesium oxide, and 2-amino-5-bromopyrimidine The ratio is 0.1:0.8:1. Under the hydrogen pressure of 0.05MPa, the reduction reaction was carri...
Embodiment 2
[0025] Add a 250ml four-necked flask to 6mol of solute and 94wt% mass concentration of sulfuric acid, adjust its temperature to 6°C, slowly add 1mol of dicyandiamide to the four-necked flask while stirring continuously, and heat up to 60°C to hydrolyze it Reaction 4h. Then cool to room temperature. Add 0.909mol 1-bromo-1-acetyl-3,3-dichloropropane into the four-necked flask, adjust the temperature to 55°C and maintain it for 1h, then raise the temperature to 60°C and maintain it for 4h. The product is extracted with toluene extractant and cooled with ice water to obtain 2-aminopyrimidine.
[0026] To the remaining cyclization reaction product after extraction, add a solvent of ethanol and water with a volume ratio of 1:5, a palladium carbon catalyst, and magnesium oxide to control the quality of the palladium carbon catalyst, magnesium oxide, and 2-amino-5-bromopyrimidine The ratio is 0.4:1.5:1. Under the hydrogen pressure of 0.2MPa, the reduction reaction was carried out a...
Embodiment 3
[0029] Add 250ml four-necked flask to sulfuric acid with 3mol of solute and 95wt% mass concentration, adjust its temperature to 5°C, slowly add 1mol of dicyandiamide into the four-necked flask while stirring continuously, and heat up to 55°C to hydrolyze it Reaction 5h. Then cool to room temperature. Add 0.556mol 1-bromo-1-acetyl-3,3-dichloropropane into the four-necked flask, first adjust the temperature to 52°C and maintain it for 1.5h, then raise the temperature to 58°C and maintain it for 5h. The product is extracted with toluene extractant and cooled with ice water to obtain 2-aminopyrimidine.
[0030] Add a solvent of ethanol and water with a volume ratio of 1:3, a palladium carbon catalyst, and magnesium oxide to the remaining cyclization reaction product after extraction, and control the quality of the palladium carbon catalyst, magnesium oxide, and 2-amino-5-bromopyrimidine The ratio is 0.3:1.1:1. Under the hydrogen pressure of 0.14MPa, the reaction was reduced at ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| flash point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com