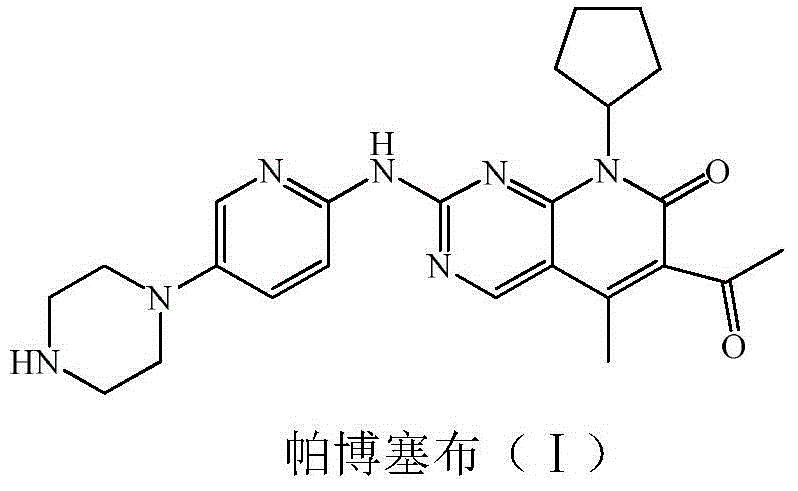
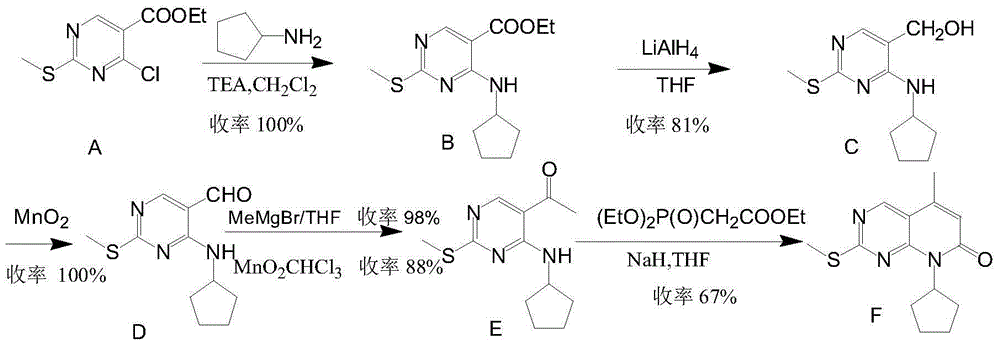
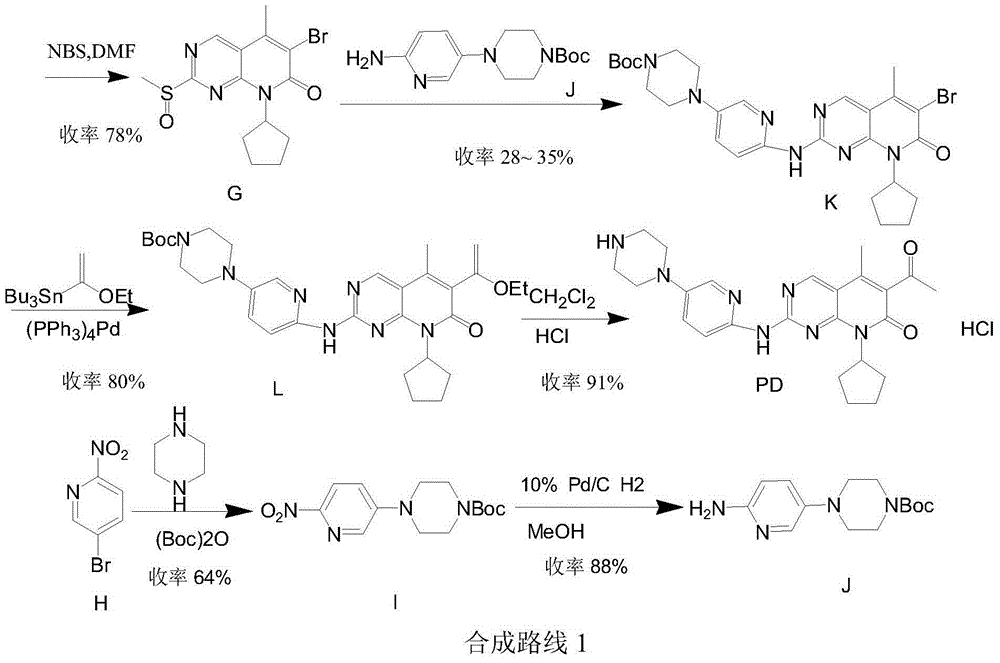
Low-cost preparation method for palbociclib
A low-cost, compound technology, applied in the field of medicine and biochemical industry, can solve problems such as increasing synthesis cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Example 1: Preparation of 2,4-dichloropyrimidine-5-(ethylenedioxy)ethylpyrimidine (Ⅲ-1)
[0075] The initial raw material 2,4-dichloro-5-cyanopyrimidine 34.8g, dissolved in 100ml tetrahydrofuran solution, warmed up to 55-60°C, began to add equimolar methylmagnesium bromide tetrahydrofuran Grignard solution dropwise, and the dropwise addition was completed After 65°C, keep warm for 3 hours, then cool down to 15-20°C, add 50mL of dilute hydrochloric acid, stir, separate layers, dry the oil layer with anhydrous sodium sulfate, recover tetrahydrofuran, and the residue is 2,4-dichloro-5-acetyl The base pyrimidine (II) was 34.4 g, and the yield was 90%.
[0076] Dissolve 34.4g of 2,4-dichloro-5-acetylpyrimidine (II) in 120mL of toluene, add 11.5g of ethylene glycol, add 0.17g of p-toluenesulfonic acid, reflux with water until a theoretical amount of water is generated , stop the reaction, cool down to room temperature, add 0.5g of sodium methoxide, add 20mL of water to wash,...
Embodiment 2
[0079] Example 2: Preparation of 5-acetyl-2-dichloro-4-cyclopentylaminopyrimidine (IV)
[0080]Dissolve the compound (III-1) of Example 1 in 100ml of NMP, add 30mL of triethylamine, add 65g of cyclopentylamine, raise the temperature to 20-25°C for 8h, and then recover the remaining cyclopentylamine, tri Ethylamine and NMP, add 50mL of water to the residue, then add 6mL of concentrated hydrochloric acid, heat up to 60-70°C, react for 2h, adjust the pH to 9-10 with sodium carbonate solution, add 100mL of ethyl acetate for extraction, extract three times, and combine the oil layers , part of the ethyl acetate was recovered by distillation, cooled, suction filtered, and dried to obtain 34.5 g of 5-acetyl-2-dichloro-4-cyclopentylaminopyrimidine (IV), with a two-step yield of 80%.
Embodiment 3
[0081] Example 3: Preparation of 5-acetyl-4-acetoacetamido-2-chloropyrimidine (V)
[0082] 5-acetyl-2-dichloro-4-cyclopentylaminopyrimidine (IV) 34.5g, dissolved in 200mL tetrahydrofuran, 5-10 ° C, dropwise added a mixed solution of 12.7g diketene and 50mL tetrahydrofuran, dropwise After completion, react at room temperature for 4h, then raise the temperature to 60°C for 2h. Part of the solvent was concentrated under reduced pressure, and the temperature was reduced by suction filtration to obtain 43.3 g of compound 5-acetyl-4-acetoacetamido-2-chloropyrimidine (V), with a yield of 93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com