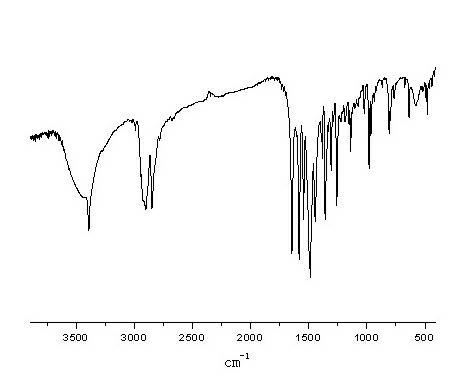
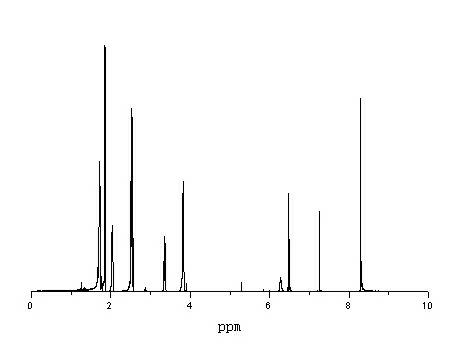
Synthesis method of N-{2-[4-(2-pyrimidyl)-1-piperazine}adamantine-1-formide
A synthetic method, pyrimidine-based technology, applied in the direction of organic chemistry, can solve the problems of increasing the concentration of serotonin, slow onset of action, etc., and achieve the effect of simple route, easy operation, and sufficient source of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 1, the synthesis of 1-(2-pyrimidine) piperazine hydrochloride
[0025] In a 100mL three-necked flask, add 3.01g of 2-chloropyrimidine and 25mL of water, stir and heat to 40°C, add 4.80g of anhydrous piperazine and 3.06g of anhydrous sodium carbonate, react at constant temperature for 4 hours, cool to room temperature, and filter to remove the precipitate , the filtrate was extracted four times with 20mL dichloromethane, the organic phases were combined, dried over anhydrous magnesium sulfate, filtered, concentrated under reduced pressure to a yellow oily liquid, the yellow oily liquid was dissolved in 40mL ether, and dry hydrogen chloride gas was introduced to precipitate white The solid was filtered and dried to obtain 4.85 g of 1-(2-pyrimidine)piperazine hydrochloride as a white solid with a yield of 92% and a melting point of 283-285°C.
[0026] 2. Synthesis of N-{2-[4-(2-pyrimidinyl)-1-piperazine]ethyl}phthalimide
[0027] In a 100mL beaker, add 5.6g of 1-(2-pyrimi...
Embodiment 2
[0034] 1, the synthesis of 1-(2-pyrimidine) piperazine hydrochloride
[0035] In a 100mL three-necked flask, add 3.01g of 2-chloropyrimidine and 40mL of water, stir and heat to 60°C, add 5.80g of anhydrous piperazine and 3.06g of anhydrous sodium carbonate in batches, react at constant temperature for 3 hours, and cool to room temperature , filtered to remove the precipitate, the filtrate was extracted four times with 20mL dichloromethane, the organic phases were combined, dried over anhydrous magnesium sulfate, filtered, concentrated under reduced pressure to a yellow oily liquid, the yellow oily liquid was dissolved in 40mL ether, and passed into a dry Hydrogen chloride gas, a white solid was precipitated, filtered, and dried to obtain 4.58 g of a white solid of 1-(2-pyrimidine)piperazine hydrochloride, with a yield of 87% and a melting point of 283-285°C.
[0036] 2. Synthesis of N-{2-[4-(2-pyrimidinyl)-1-piperazine]ethyl}phthalimide
[0037]In a 100mL beaker, add 5.6g of ...
Embodiment 3
[0044] 1, the synthesis of 1-(2-pyrimidine) piperazine hydrochloride
[0045] In a 100mL three-necked flask, add 3.01g of 2-chloropyrimidine and 50mL of water, stir and heat to 80°C, add 7.80g of anhydrous piperazine and 3.06g of anhydrous sodium carbonate in batches, react at constant temperature for 1h, and cool to room temperature , filtered to remove the precipitate, the filtrate was extracted four times with 20mL dichloromethane, the organic phases were combined, dried over anhydrous magnesium sulfate, filtered, concentrated under reduced pressure to a yellow oily liquid, the yellow oily liquid was dissolved in 40mL ether, and passed into a dry Hydrogen chloride gas precipitated a white solid, filtered and dried to obtain 4.22 g of 1-(2-pyrimidine)piperazine hydrochloride as a white solid with a yield of 80.1% and a melting point of 283-285°C.
[0046] 2. Synthesis of N-{2-[4-(2-pyrimidinyl)-1-piperazine]ethyl}phthalimide
[0047] In a 100mL beaker, add 5.6g of 1-(2-pyri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com