Synthesis process of 2-chloropyrimidin
A synthesis process, a technology of chloropyrimidine, which is applied in the field of synthesis process for preparing 2-chloropyrimidine, can solve the problems of low product purity and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
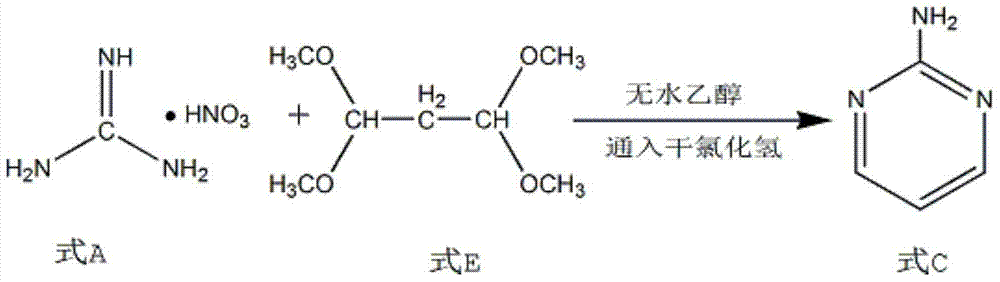
[0015] Add absolute ethanol and 1.2mol guanidine nitrate into a 250ml four-necked flask, continuously feed dry hydrogen chloride gas into the absolute ethanol and keep stirring, and after adjusting the temperature to 10-20°C, add 1 mol of 1,1,3,3-tetramethoxypropane, the temperature of the reaction system was stabilized at 20°C, and the cyclization reaction was carried out for 6 hours. After the reaction is complete, collect the formed precipitate by filtration, add the precipitate into water and add sodium hydroxide solution to adjust the pH to 9, add toluene for dehydration and separate the clear liquid while it is hot, extract the solid with toluene, combine the organic phases, and filter by cold analysis. The fine product 2-aminopyrimidine was obtained.
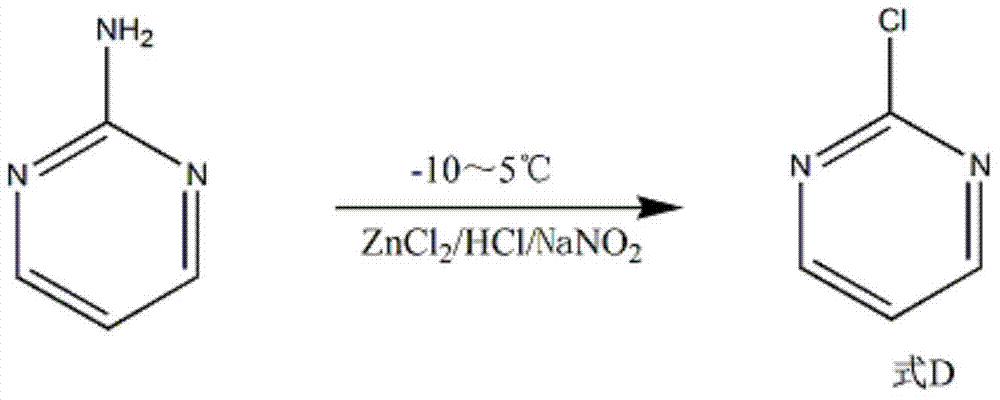
[0016] Add 2.5mol of hydrochloric acid and 80g of dichloromethane into a 500mL reactor, and then add 1mol of 2-aminopyrimidine under stirring. Add 3.2mol ZnCl at 10~15℃ 2 , After reacting for 30min, a dark yellow suspen...
Embodiment 2
[0018] Add absolute ethanol and 1.8mol guanidine nitrate to a 250ml four-necked flask, continuously feed dry hydrogen chloride gas into the absolute ethanol and keep stirring, and after adjusting the temperature to 10-20°C, add 1 mol of 1,1,3,3-tetramethoxypropane, the temperature of the reaction system was stabilized at 30°C, and the cyclization reaction was carried out for 4 hours. After the reaction is completed, collect the formed precipitate by filtration, add the precipitate to water and add sodium hydroxide solution to adjust the pH to 10, add toluene for dehydration, separate the clear liquid while hot, extract the solid with toluene, combine the organic phases, and filter by cold analysis. The fine product 2-aminopyrimidine was obtained.
[0019] Add 3.5mol of hydrochloric acid and 80g of dichloromethane into a 500mL reactor, and then add 1mol of 2-aminopyrimidine under stirring. Add 4mol ZnCl at 10~15℃ 2 , After reacting for 60min, a dark yellow suspension was gene...
Embodiment 3
[0021] Add absolute ethanol and 1.5mol guanidine nitrate into a 250ml four-necked flask, continuously feed dry hydrogen chloride gas into the absolute ethanol and keep stirring, and after adjusting the temperature to 10-20°C, add 1 mol of 1,1,3,3-tetramethoxypropane, the temperature of the reaction system was stabilized at 25°C, and the cyclization reaction was carried out for 5 hours. After the reaction is complete, collect the formed precipitate by filtration, add the precipitate to water and add sodium hydroxide solution to adjust the pH to 9.5, add toluene for dehydration, separate the clear liquid while hot, extract the solid with toluene, combine the organic phases, and filter by cold analysis. The fine product 2-aminopyrimidine was obtained.
[0022] Add 3 mol of hydrochloric acid and 80 g of dichloromethane into a 500 mL reactor, and then add 1 mol of 2-aminopyrimidine under stirring. Add 3.5mol ZnCl at 10~15℃ 2 , After reacting for 45min, a dark yellow suspension wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com