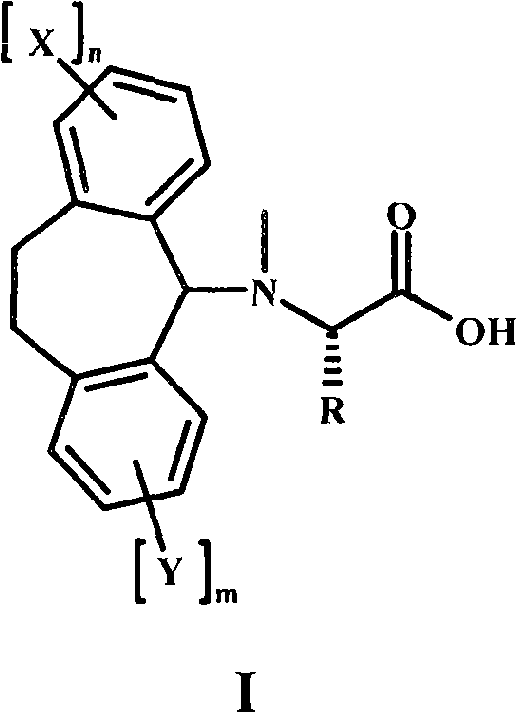
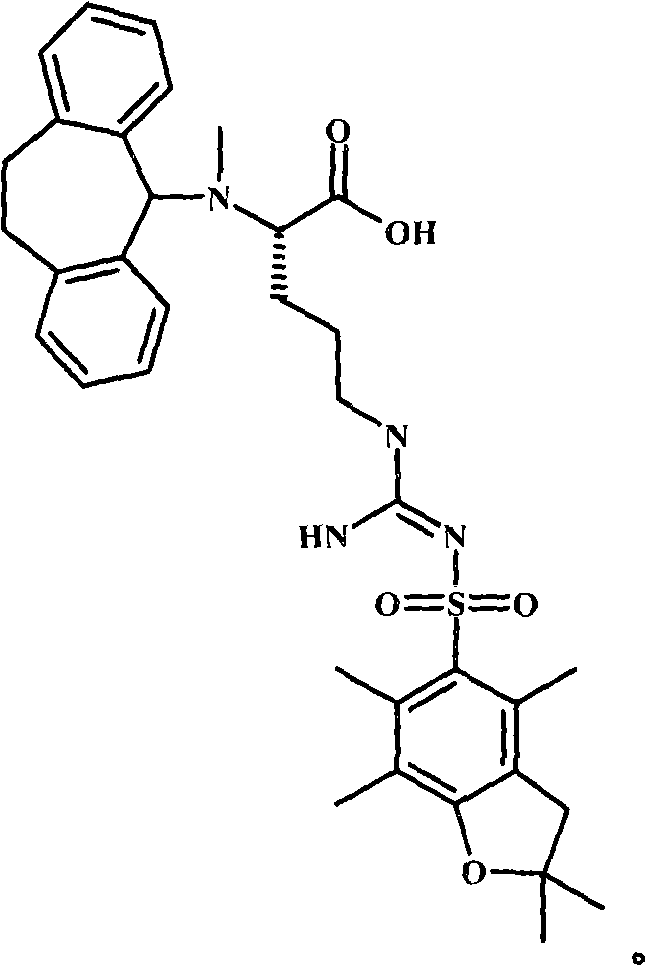
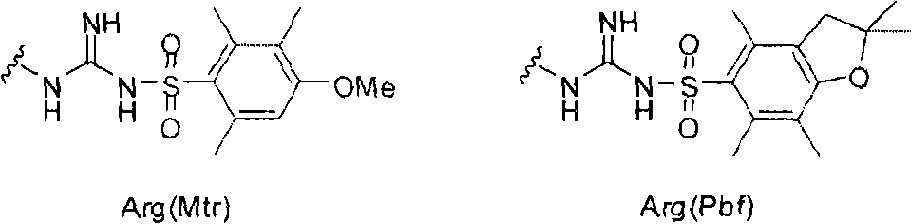Alpha-n-methylation of amino acids
A technology for amino acids and amino acid side chains, applied in organic chemistry, peptide preparation methods, organic chemistry methods, etc., can solve problems such as complex chromatographic purification steps, inability to penetrate catalyst particles, low yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0133] Step 1 - Dbs Protection:
[0134] 0.01mol H was obtained after Fmoc deprotection 2 N-Arg(Pbf)-Tyr(tBu)-Rink-Ahx-resin, the resin was washed appropriately (2×DMF, 2×CH 2 Cl 2 , 2×DMF) and drained. 0.05 mol Dbs-Cl was dissolved in 150 mL DMF, and 50 mL 1:1 DIEA:toluene was added thereto. This solution was then added to the above resin, which was then stirred for 4 hours. At this point ninhydrin was negative. The resin was then washed (5 x DMF) and suspended in DMF for the next step.
[0135] 2-methylation:
[0136] The following methylating reagent solutions were prepared immediately before use: 300 mL NMP, 80 mL THF, 20 mL formaldehyde solution (0.246 mol), 4 mL acetic acid.
[0137] The resin from step 1 was sucked dry, and then the above methylation reagent solution was added. The resin was then stirred for 15 minutes to form the methylimine. Then weigh 0.1mol (6.2g) NaCNBH 3 , added to the stirred peptide resin in one portion via a powder funnel, with a mini...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com