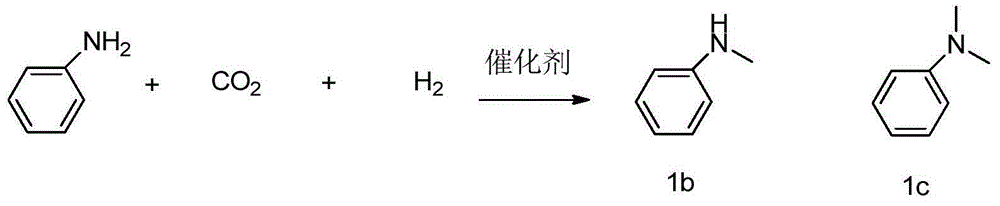
Preparation method of N-methylamine compound
A technology of secondary amine compounds and compounds, applied in the field of preparation of N-methylamine compounds, which can solve the problems of complex preparation process, difficult separation of products, high production cost, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Embodiment 1 (Al 2 o 3 carrier preparation)
[0080] Take a certain amount of aluminum nitrate and dissolve it in deionized water (0.8 mol / L), and add 2.6 mol / L ammonia solution while stirring until the pH value of the solution reaches about 9. Then stir at room temperature for 4 hours, then age for 2 hours, filter and wash for more than 3 times until there is no nitrate ion in the solution, and wash with ethanol for the last time. Al 2 o 3 carrier.
Embodiment 2
[0081] Embodiment 2 (Au / Al 2 o 3 Catalyst Preparation)
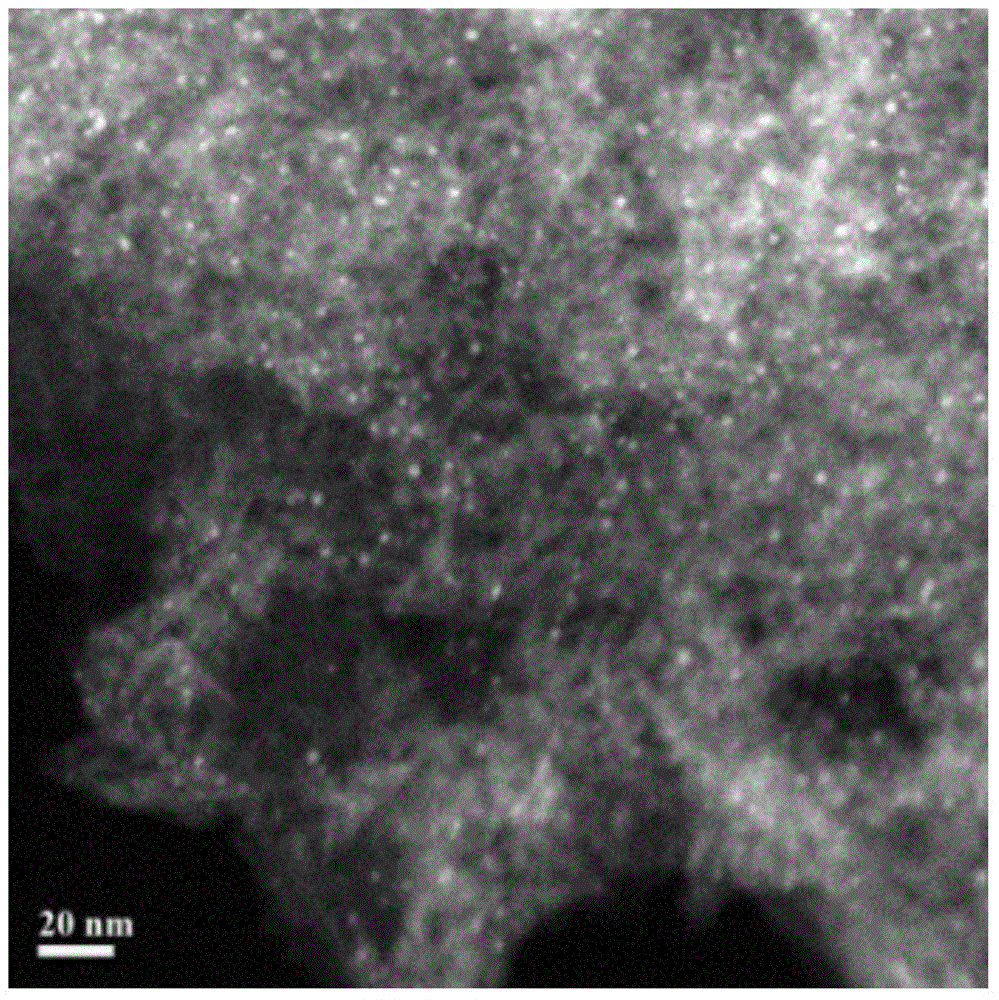
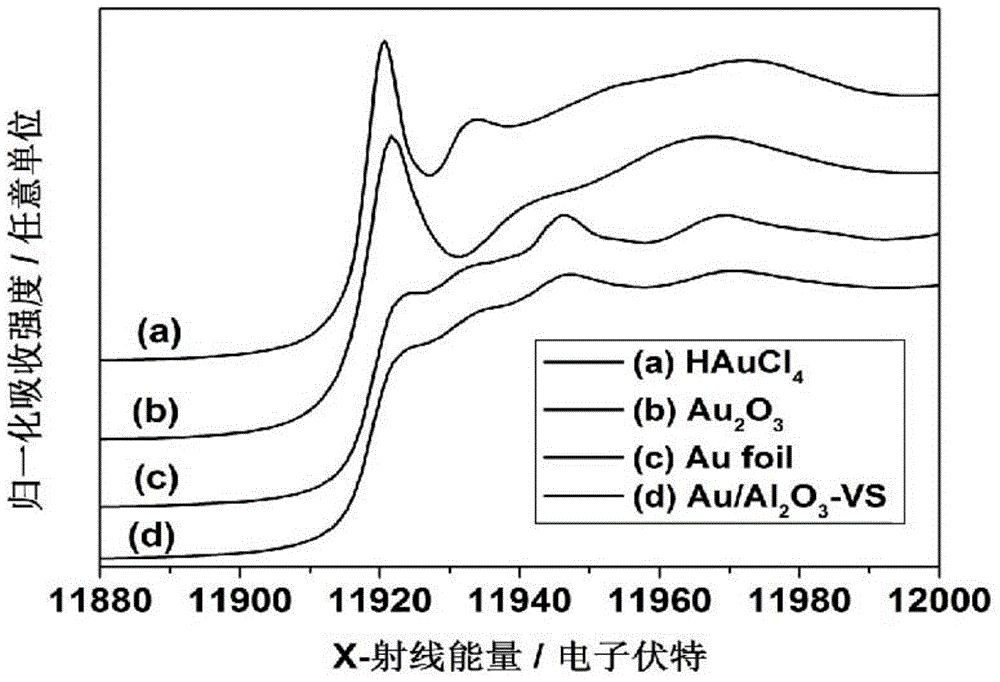
[0082] Au / Al with a theoretical loading of 1wt% 2 o 3 The catalyst is taken as an example, prepared by urea deposition precipitation method (DPU). The catalyst was prepared in a beaker wrapped in tin foil to avoid light. Under the condition of stirring at room temperature, the Al prepared in 1g embodiment 1 2 o 3 The carrier was added to 100 mL of chloroauric acid aqueous solution containing 0.48 mmol / L* (calculated as Au). Then add a certain amount of urea (urea / Au=200, molar ratio). The temperature of the water bath was then raised to 80°C under constant stirring and kept at this temperature for 6 hours. After cooling down to room temperature, filter and wash for more than 3 times until there is no chloride ion in the solution. After vacuum drying at 350°C using 5vol.% H 2 / Ar reduction for 2 hours, the heating rate was 5°C / min, and a powdered catalyst sample was prepared. The actual gold loading of the cata...
Embodiment 3
[0085] Embodiment 3 (Pt / Al 2 o 3 , Rh / Al 2 o 3 , Ir / Al 2 o 3 Catalyst Preparation)
[0086] Pipette an appropriate amount of chloroplatinic acid, rhodium nitrate, and chloroiridic acid aqueous solution in three beakers with 100 mL of deionized water respectively, and then add 1 gram of Al 2 o 3 The powder was uniformly stirred for 2 hours at 80°C, and then the water was evaporated to dryness at 90°C. The obtained solid was transferred into a watch glass and dried in a vacuum oven for 24 hours. The sample was placed in a muffle furnace for 400 ℃ roasting for 2 hours, and finally the roasted sample in 5vol.%H 2 / Ar atmosphere for 2 hours, the heating rate is 5° C. / min, and the powdery supported noble metal catalyst sample is prepared. Expressed as: 1%Pt / Al 2 o 3 , 1%Rh / Al 2 o 3 , 1%Ir / Al 2 o 3 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com