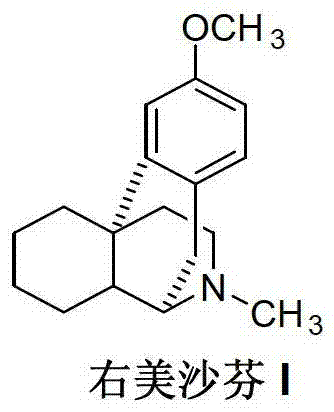
Preparation method of dextromethorphan
A technology of dextromethorphan and benzyl, which is applied in the field of preparation of dextromethorphan, can solve problems such as low yield of dextromethorphan and difficulty in separation, and achieve the effects of promoting economic and technological development, reducing production costs and improving quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
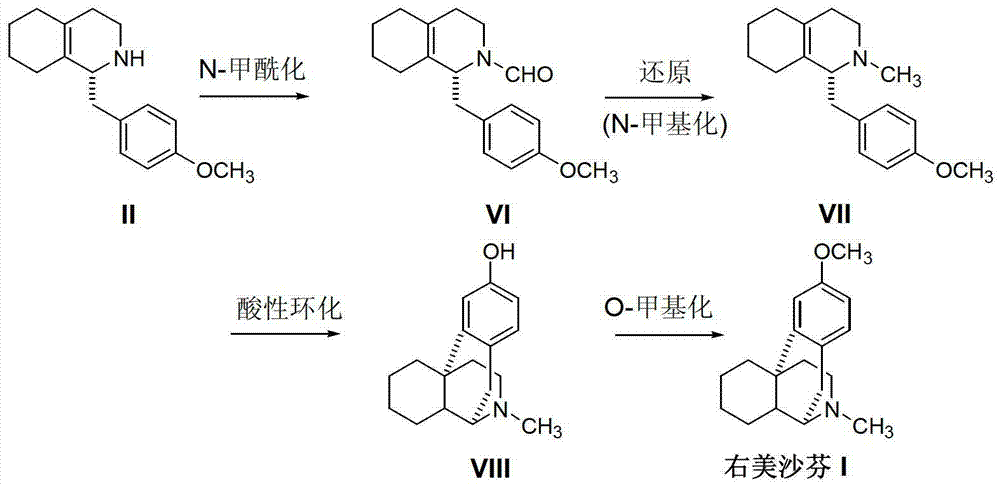
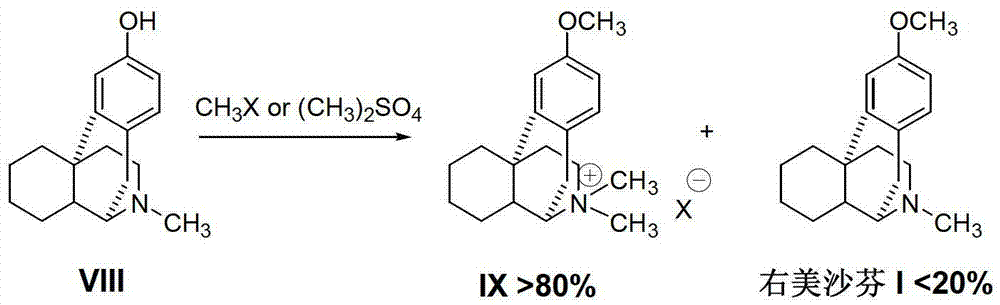
[0025] How to prepare dextromethorphan simply and conveniently using the above invention will be described below through a specific preparation process and method, wherein the acidic cyclization reaction can refer to EP0834505A1 and "Pharmaceutics Today" 2008 Volume 18 No. 4 Page 63.
[0026] N-benzylation reaction: Add the intermediate (+)-1-(4-methoxy)benzyl-1,2,3,4,5,6,7,8-eta Hydroisoquinoline (II) (25.7g, 0.1mol), benzyl bromide (20.4g, 0.12mol) and 200mL of 95% ethanol, start stirring, add solid potassium carbonate (13.8g, 0.1mol) in batches, and heat up to 80-85 DEG C, keep stirring under this temperature and react for 8 hours, TLC detects that the reaction ends. Concentrate under reduced pressure to half of the total volume, and cool down to room temperature. Solids are precipitated, and the filter cake is washed with n-hexane. Methanol and water (3:1) recrystallized to obtain off-white solid (+)-1-(4-methoxy)benzyl-N-benzyl-1,2,3,4,5,6,7, 31.4 g of 8-octahydroisoqui...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com