Preparation method of arecoline
A technology of arecoline and organic bases, applied in the direction of organic chemistry, can solve the problems of expensive methyl iodide, high processing costs, long process routes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
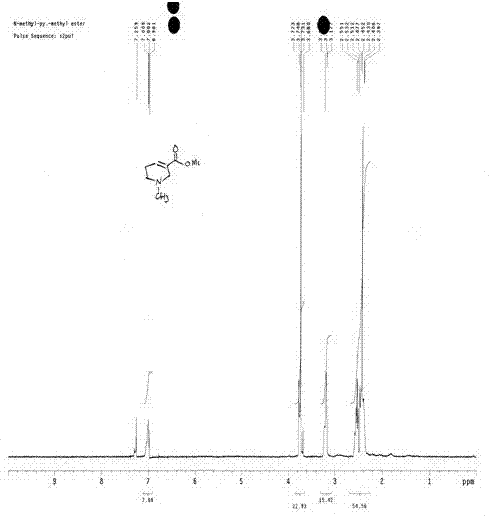
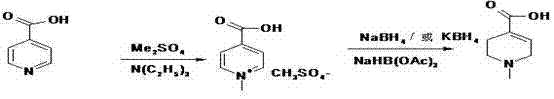
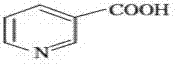
[0032] Add 50g (0.406mol) of nicotinic acid and 45.2g (0.447mol) of triethylamine into 500ml of toluene, raise the temperature to 70°C, add 112.6g (0.894mol) of dimethyl sulfate dropwise at this temperature, after the addition is complete, raise the temperature to 100 °C, react for 3Hr, and cool to room temperature. Since the hydroboration reaction is carried out in a mixed solution of benzene and water (document J.Org.chem.1955, 1761-1766), the reactant in the previous step does not need to be processed to obtain pure N-methyl nicotinic acid methyl ester or N- Methyl nicotinate monomethyl sulfate can be hydroborated. Add 500ml of water to the system formed after the above reaction, and add 31g of NaBH at 35-38°C 4 (0.82mol), added in batches for about 1 hr, and reacted at room temperature for 4 hours after the addition. The whole reaction process is shown in formula VI:
[0033]
[0034] (Formula VI).
[0035] Then stand for stratification, the organic phase is the tol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com