Arylboronic acid derivatives and preparation method thereof
A technology of aryl boronic acid and derivatives, applied in the field of aryl boronic acid derivatives and their preparation, can solve problems such as insufficient environmental protection of organic flame retardants, and achieve the effect of solving high toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 3
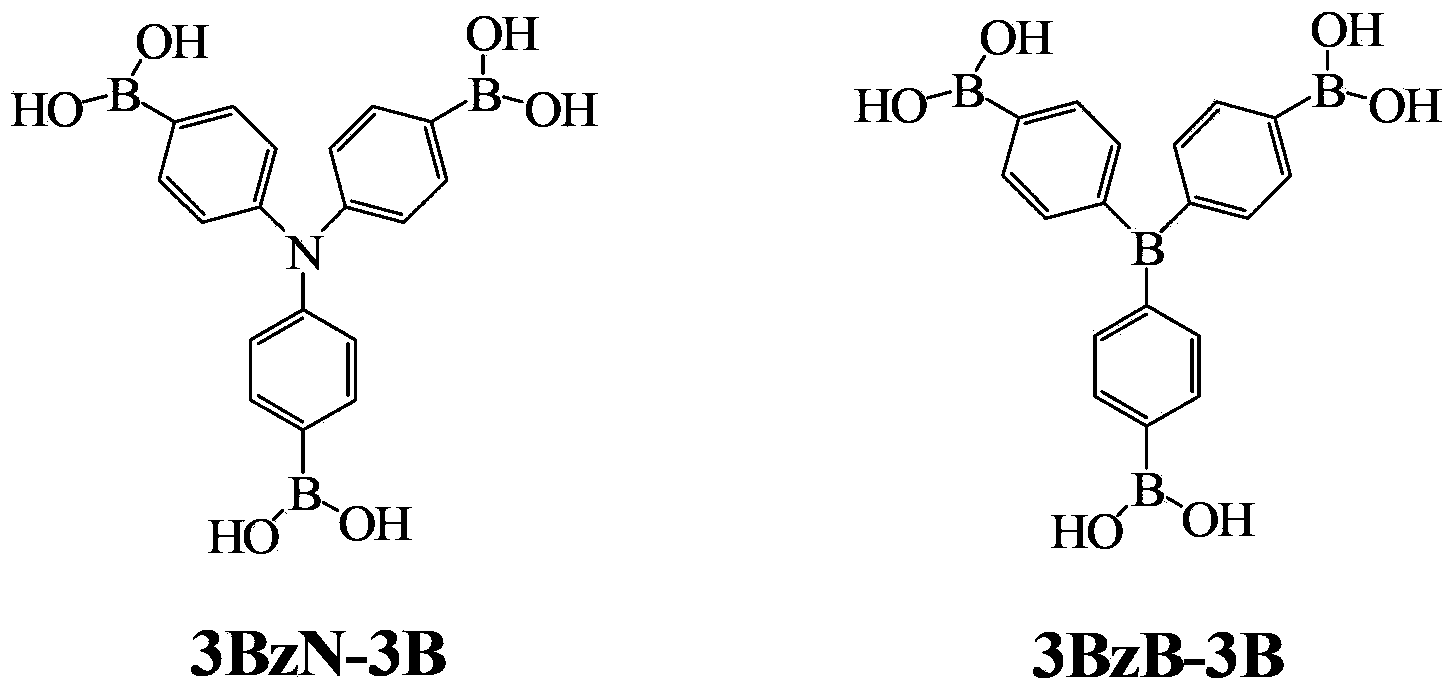
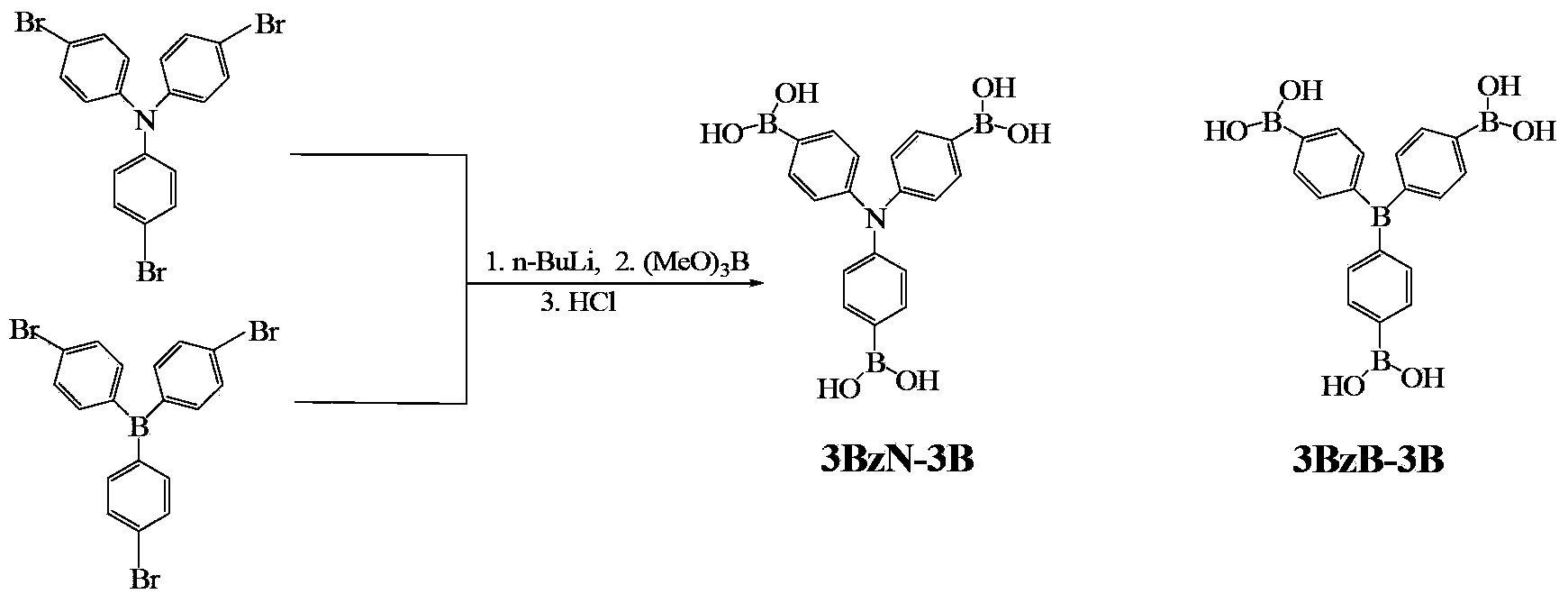
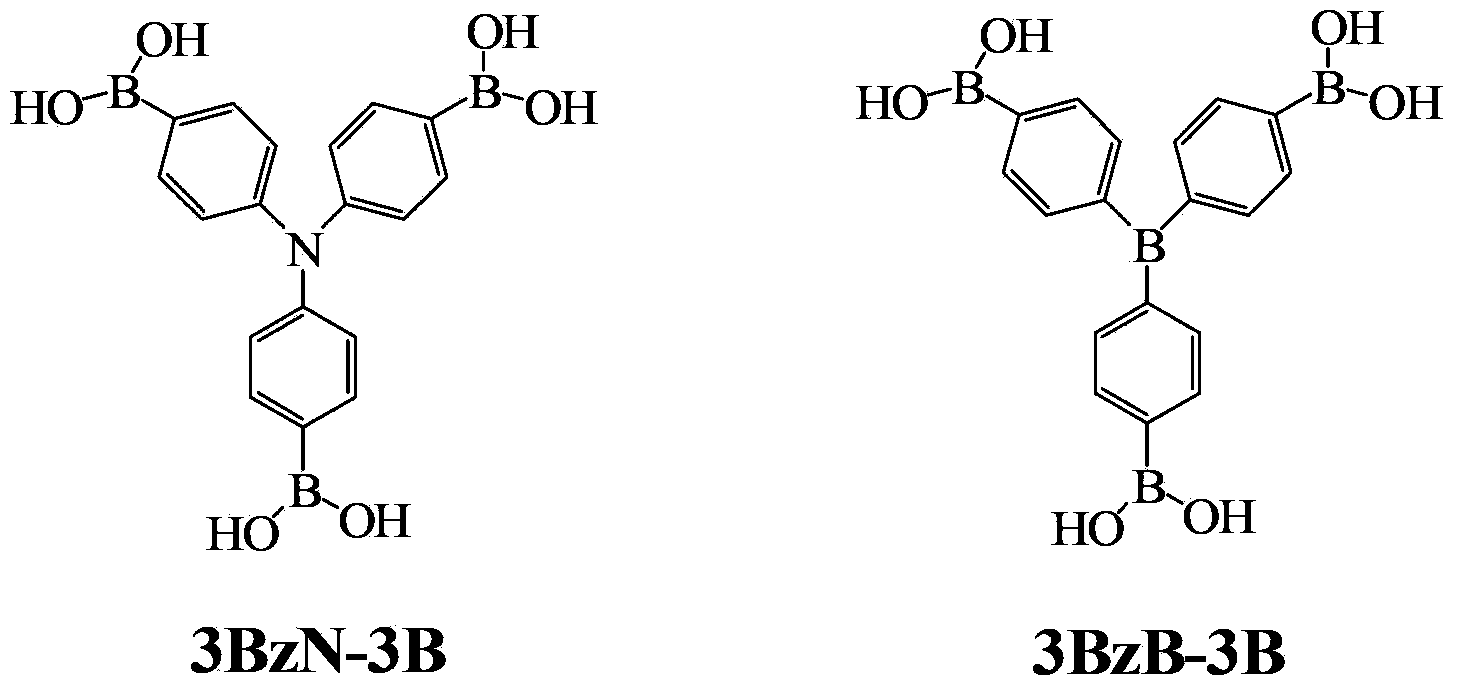
[0022] Example 1 Preparation of Tris(4,4',4"-triboronic acid-phenyl)amine (3BzN-3B)
[0023] In the first step, under the protection of nitrogen, add n-butyllithium dropwise to the tetrahydrofuran solution of tris(4,4',4"-tribromo-phenyl)amine, control the reaction temperature at -78°C, stir for 1 hour, and then rise to Stir at room temperature for 45 minutes;
[0024] In the second step, the temperature of the reaction liquid obtained in the first step is lowered to -78 ° C, and trimethyl borate is added dropwise therein, stirred for 45 minutes, then warmed up to room temperature and stirred for about 10 hours;
[0025] In the third step, a rotary evaporator is used to remove tetrahydrofuran and unreacted trimethyl borate in the reaction liquid of the second step to obtain a solid product. Dissolve the solid product with dichloromethane, then add saturated aqueous ammonium chloride for extraction, collect the organic phase, dry with anhydrous sodium sulfate and filter, and r...
Embodiment 2 3
[0031] Example 2 Preparation of Tris(4,4',4"-triboronic acid-phenyl)amine (3BzN-3B)
[0032] In the first step, under the protection of nitrogen, add n-butyllithium dropwise to the tetrahydrofuran solution of tris(4,4',4"-tribromo-phenyl)amine, control the reaction temperature at -78°C, stir for 2 hours, and then rise to Stir at room temperature for 36 minutes;
[0033] In the second step, the temperature of the reaction liquid obtained in the first step is lowered to -78°C, and trimethyl borate is added dropwise thereto, stirred for 55 minutes, then warmed up to room temperature and stirred for about 10 hours;
[0034] In the third step, a rotary evaporator is used to remove tetrahydrofuran and unreacted trimethyl borate in the reaction liquid of the second step to obtain a solid product. Dissolve the solid product with dichloromethane, then add saturated aqueous ammonium chloride for extraction, collect the organic phase, dry with anhydrous sodium sulfate and filter, and re...
Embodiment 3 3
[0036] Example 3 Preparation of Tris(4,4',4"-triboronic acid-phenyl)amine (3BzN-3B)
[0037] In the first step, under the protection of nitrogen, add n-butyllithium dropwise to the tetrahydrofuran solution of tris(4,4',4"-tribromo-phenyl)amine, control the reaction temperature at -78°C, stir for 3 hours, and then rise to Stir at room temperature for 30 minutes;
[0038] In the second step, the temperature of the reaction liquid obtained in the first step is lowered to -78 ° C, and trimethyl borate is added dropwise therein, stirred for 80 minutes, then warmed up to room temperature and stirred for about 10 hours;
[0039] In the third step, a rotary evaporator is used to remove tetrahydrofuran and unreacted trimethyl borate in the reaction liquid of the second step to obtain a solid product. Dissolve the solid product with dichloromethane, then add saturated aqueous ammonium chloride for extraction, collect the organic phase, dry with anhydrous sodium sulfate and filter, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com