Preparation method for 9,9-diaryl thiophene xanthene-10,10-dioxide
A technology of dioxide and thiaxanthene, which is applied in the new preparation field, can solve the problems of human, environmental hazard, high production cost, long synthesis route and the like, and achieves the effects of easy industrialization, low cost and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
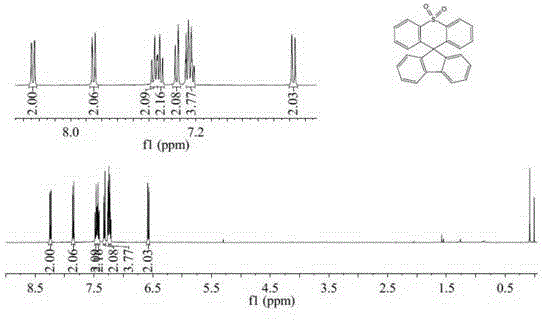
[0031] Example 1: Preparation of spirofluorenethiaxanthene-10,10-dioxide
[0032]
[0033] Weigh diphenyl sulfone (4.75g, 0.022mol, 1.05eq) and methyl 2-biphenylcarboxylate (4.4g, 0.021mol, 1eq) and put them into a two-necked flask and a constant pressure dropping funnel respectively, and use a dry Diphenylsulfone was dissolved in 120 mL of tetrahydrofuran. Under nitrogen protection and a dry ice-ethanol bath, slowly add 1.6M n-butyllithium hexane solution (28.5mL, 0.045mol, 2.2eq) dropwise, keep the reaction at low temperature for 3h, and quickly add methyl 2-biphenylcarboxylate in tetrahydrofuran solution; react at room temperature for 24 hours, after the reaction, add saturated ammonium chloride solution for hydrolysis, extract with dichloromethane, dry, and remove the solvent; put the obtained tertiary alcohol into a two-necked flask, add 100mL of glacial acetic acid, and heat Reflux to dissolve, take 0.5mL of concentrated sulfuric acid and add it to the reaction solut...
example 2
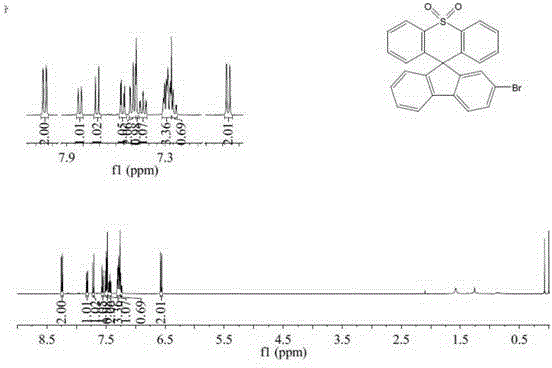
[0034] Example 2: Preparation of 2-bromospirofluorenethiaxanthene-10,10-dioxide
[0035]
[0036] Weigh diphenyl sulfone (3.93g, 0.018mol, 1.1eq) and ethyl 2-(4'-bromobiphenyl) formate (5.0g, 0.0164mol, 1eq) and put them into a two-necked flask and constant pressure drop solution respectively. In the funnel, dissolve diphenyl sulfone with 80 mL of dry tetrahydrofuran. Under nitrogen protection and a dry ice-ethanol bath, slowly add 1.6M n-butyllithium hexane solution (22.5mL, 0.036mol, 2.2eq) dropwise, maintain the low temperature reaction for 3h, and quickly add 2-(4'-bromobiphenyl ) tetrahydrofuran solution of ethyl formate; react at room temperature for 20h, after the reaction is over, add saturated ammonium chloride solution for hydrolysis, extract with dichloromethane, dry, and remove the solvent; put the obtained tertiary alcohol into a two-necked flask, add 80mL glacial acetic acid, and heated to reflux to dissolve, take 0.5mL of concentrated sulfuric acid and add t...
example 3
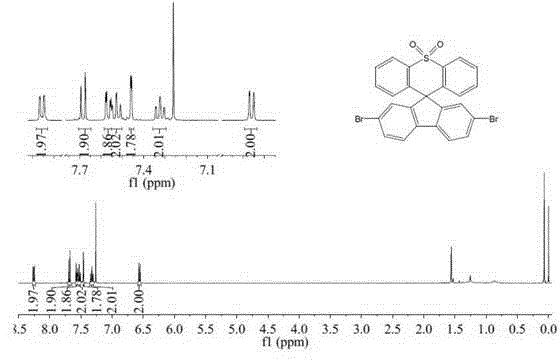
[0037] Example 3: Preparation of 2,7-dibromospirofluorenethiaxanthene-10,10-dioxide
[0038] There are many schemes for the synthesis of 2,7-dibromospirofluorenethiaxanthene-10,10-dioxide, and the most economical and convenient synthesis scheme is as follows:
[0039]
[0040] The specific process of spirofluorenethiaxanthene-10,10-dioxide is as in Example 1. Take spirofluorene sulfone xanthene (1.9g, 0.005mol) and a catalytic amount of iron powder (0.036g) into a two-necked flask, and dissolve it with 50mL of chloroform; take liquid bromine (1.84g, 0.0115mol) and inject it into the flask , reacted under reflux conditions for 14 hours; after the reaction, add sodium bisulfite solution to the reaction flask, and stir for 30 minutes, extract with dichloromethane, combine organic phases, dry over anhydrous magnesium sulfate, suction filter, spin dry and pass through the column After separation, 2.5 g of white solid product 2,7-dibromospirofluorene sulfone xanthene was obtaine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com