Non-metallocene catalyst and preparation method and application thereof
A transition metal, non-precene technology, applied in the field of non-precene transition catalysts and their preparation
Inactive Publication Date: 2010-10-27
INST OF CHEM CHINESE ACAD OF SCI
View PDF2 Cites 9 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, there is currently no non-pre-transition metal catalyst that can use polar solvents for ethylene polymerization
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
preparation example Construction
Embodiment 1
Embodiment 2
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
Login to View More
Abstract
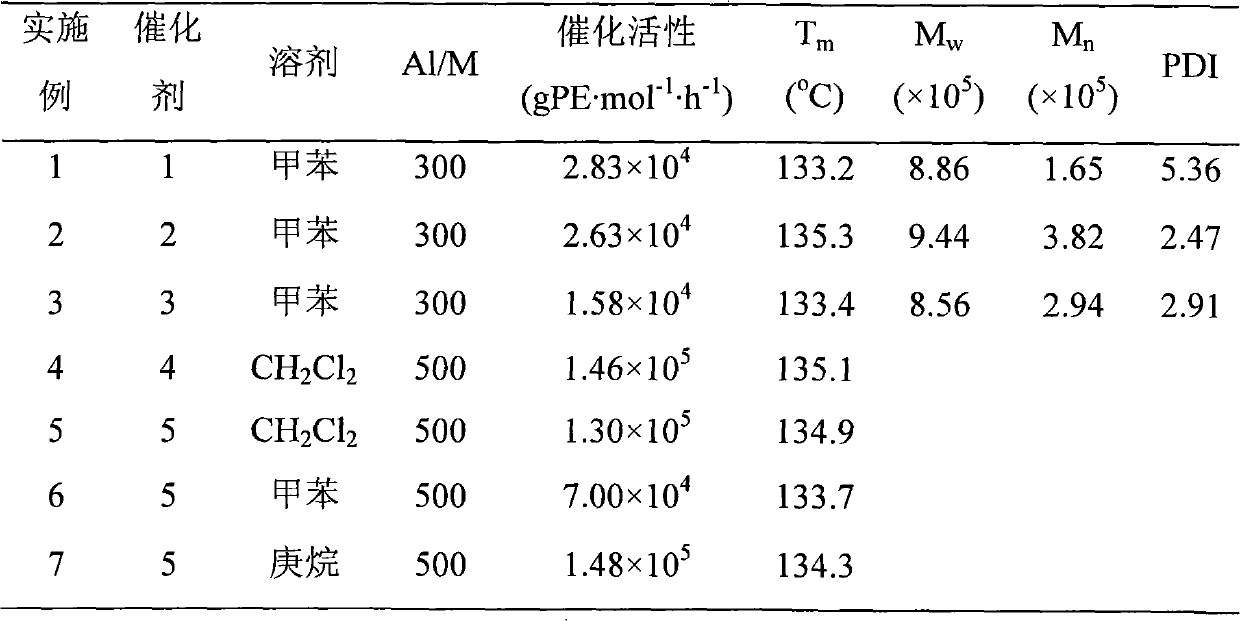
The invention discloses a non-metallocene catalyst and a preparation method and the application thereof. The general structural formula of the non-metallocene catalyst is as shown in formula I. The preparation method of the catalyst comprises the following steps: (1) pyridine or pyridone formaldehyde containing a substituent and amino phenol or amino thiophenol containing the other substituent are put into organic solvent to have reflux reaction and obtain a ligand; and (2) first the organic solution of the ligand obtained in step (1) reacts with butyl lithium, is added with metal halide to react and obtain the non-metallocene catalyst after reaction. When the non-metallocene catalyst is used for ethylene polymerization, not only dichloromethane with polarity but also non-polar toluene and heptane can be selected as the solvent during the ethylene polymerization process, and higher activity is also achieved during the process; and the polyethylene has high molecular weight, and the weight-average molecular weight is 800,000 to 1 million(Formula I is shown as the accompanying drawing.).
Description
technical field The present invention relates to a non-procene transition metal catalyst and its preparation method and application, in particular to an imine-type non-procene transition metal catalyst with three coordination of N, N, O and N, N, S and its preparation method and its application in Applications in the polymerization of ethylene. Background technique Non-metallocene olefin polymerization catalysts are ring-free and contain monodentate or multidentate ligands whose coordination atoms are carbon, nitrogen, oxygen, sulfur, phosphorus, etc. The central metal includes all transition elements and some metals of the main group organic compounds. In recent years, catalytic polymerization based on transition metal compounds has outstanding advantages over other polymerization processes, such as precise control of polymer chain structure, milder polymerization conditions in terms of temperature and pressure, etc. This field involves organic Chemistry, inorganic chemis...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C08F4/76C08F4/659C08F10/02
CPCY02P20/52
Inventor 董金勇温笑菁
Owner INST OF CHEM CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com