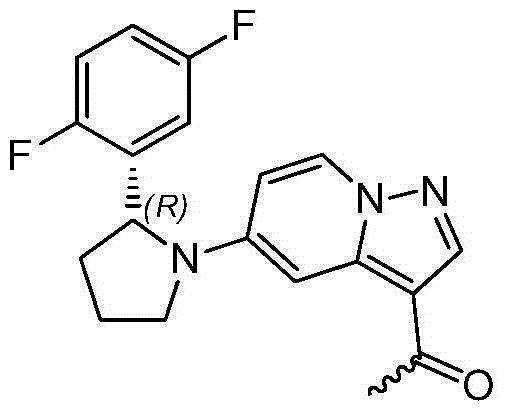
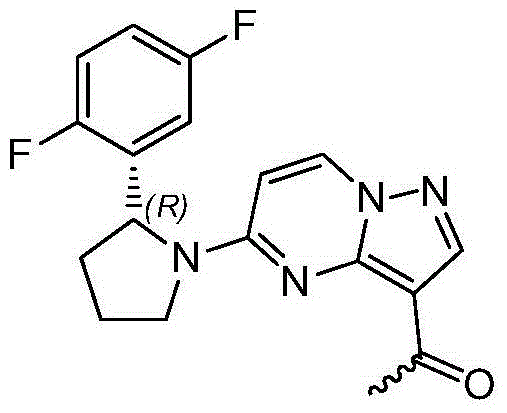
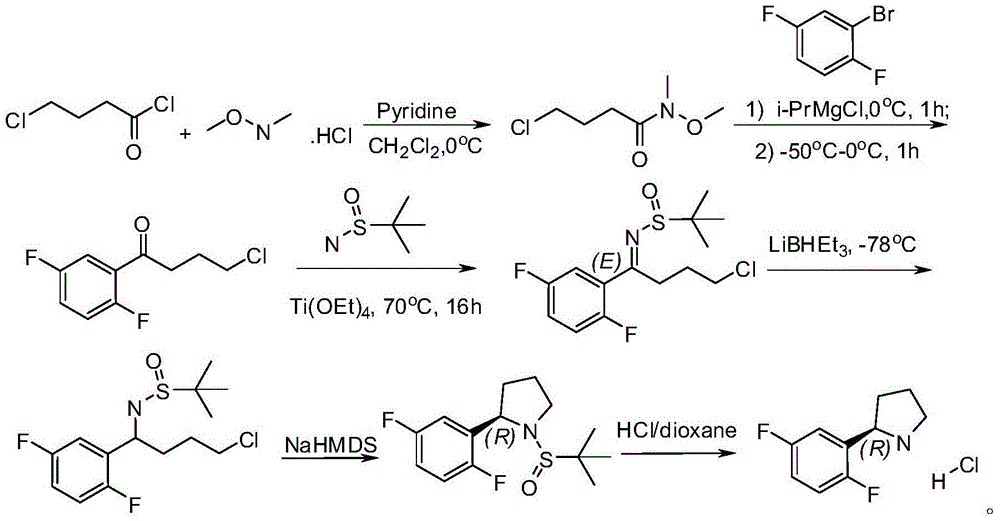
Method for preparing 2R-(2, 5-difluorophenyl) pyrrolidine hydrochloride
A technology of pyrrolidine hydrochloride and dimethylhydroxylamine hydrochloride, which is applied in the field of chemical drug synthesis, can solve the problems of harsh reaction conditions, high cost of raw materials, and high cost of reagents, so as to reduce reagent costs, increase yields, and add materials convenient effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Compound 2 was synthesized.
[0039]
[0040] N, O-dimethylhydroxylamine hydrochloride (30.4g, 0.31mol) was dissolved in dichloromethane (500ml), cooled in an ice bath, stirred, added triethylamine (65g, 0.64mol), and then added dropwise compound 1 ( 40g, 0.28mol), the internal temperature is controlled between 0-6 degrees Celsius. After the addition was complete, the reaction was carried out at room temperature for 3 hours and quenched with dilute hydrochloric acid (1M). The organic layer was separated, and the organic layer was successively washed with saturated NaHCO 3 (100ml) and saturated saline (100ml) each washed once, with anhydrous Na 2 SO 4 After drying, the solvent was removed under reduced pressure to obtain compound 2 (45 g, 96%, light brown liquid).
Embodiment 2
[0042] Compound 3 was synthesized.
[0043]
[0044] 1,4-difluorobenzene (12g, 0.105mol) was dissolved in tetrahydrofuran (50ml), cooled to -70°C,
[0045] Slowly add n-butyllithium (46.4ml, 2.5M, 0.116mol) dropwise, control the internal temperature between -70°C and -60°C, and complete the dropwise addition in 2 hours. The reaction solution was stirred at -70°C for 1 hour, and then a solution of compound 2 in tetrahydrofuran (19 g, 0.116 mol, dissolved in 30 ml tetrahydrofuran) was added dropwise, and the addition was completed in half an hour. The reaction continued to stir at -70°C for 1 hour, raised to room temperature, and stirred for another half an hour, then quenched the reaction with saturated ammonium chloride (50ml), extracted with methyl tert-butyl ether (100ml×2), and then washed with saturated Brine (30ml) was backwashed once, dried with anhydrous sodium sulfate, concentrated to dryness under reduced pressure, and separated by column chromatography (petroleum...
Embodiment 3
[0047] 1,4-Difluorobenzene (12g, 0.105mol) was dissolved in ether (50ml), cooled to -50°C, slowly added n-butyl lithium (46.4ml, 2.5M, 0.116mol) dropwise, and the internal temperature was controlled at -60 Between ℃ and -50℃, the dropwise addition was completed in 2 hours. The reaction solution was stirred at -50°C for 1 hour, then a diethyl ether solution of compound 2 (19 g, 0.116 mol, dissolved in 30 ml diethyl ether) was added dropwise, and the addition was completed in half an hour. The reaction continued to stir at -50°C for 1 hour, warmed to room temperature, and stirred for another half an hour, then quenched with saturated ammonium chloride (50ml), extracted with methyl tert-butyl ether (100ml×2), and then washed with saturated Brine (30ml) was backwashed once, dried with anhydrous sodium sulfate, concentrated to dryness under reduced pressure, and separated by column chromatography (petroleum ether / ethyl acetate=40 / 1) to obtain compound 3 (15g, 65.2%, brown liquid )...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com