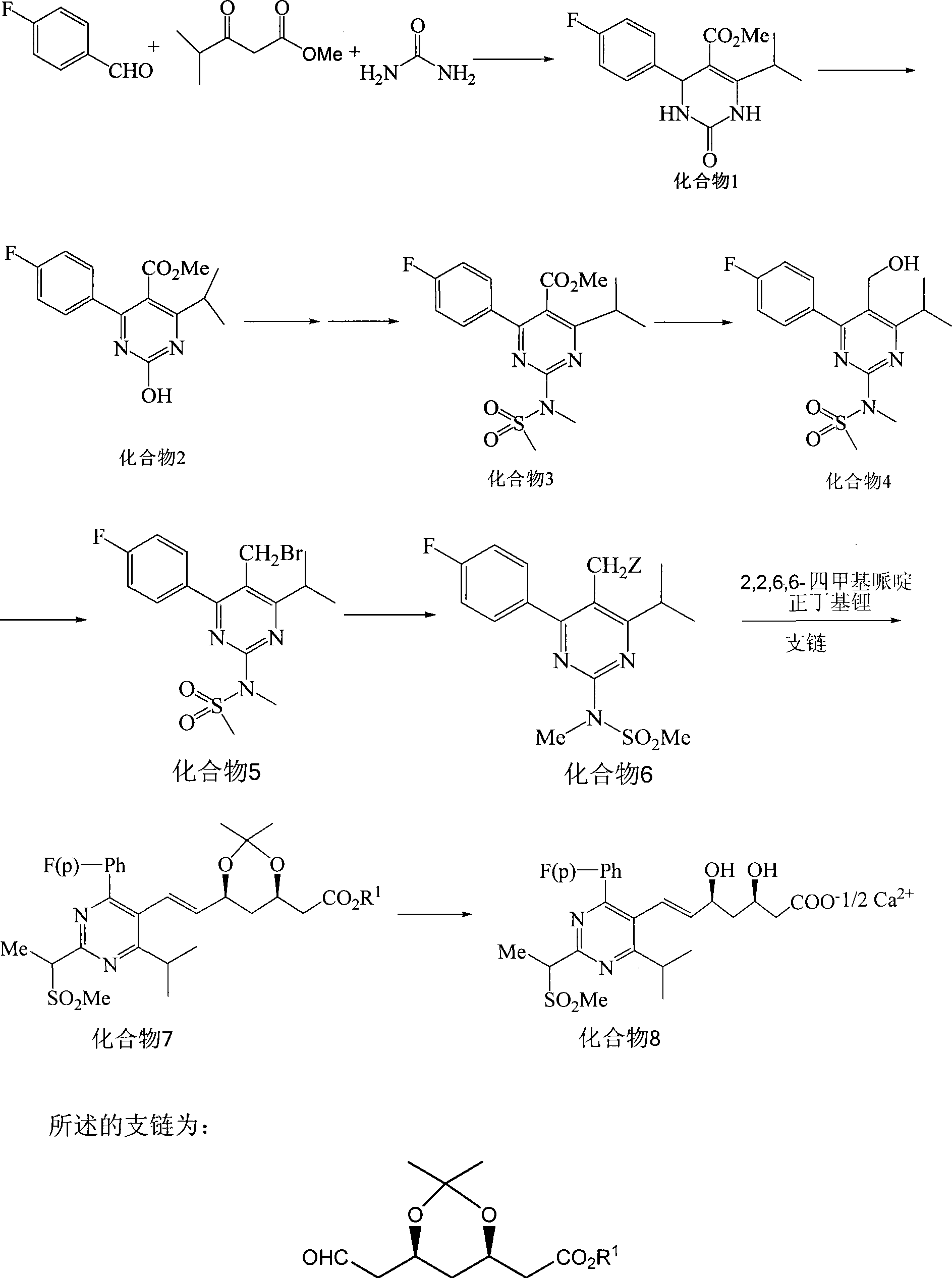
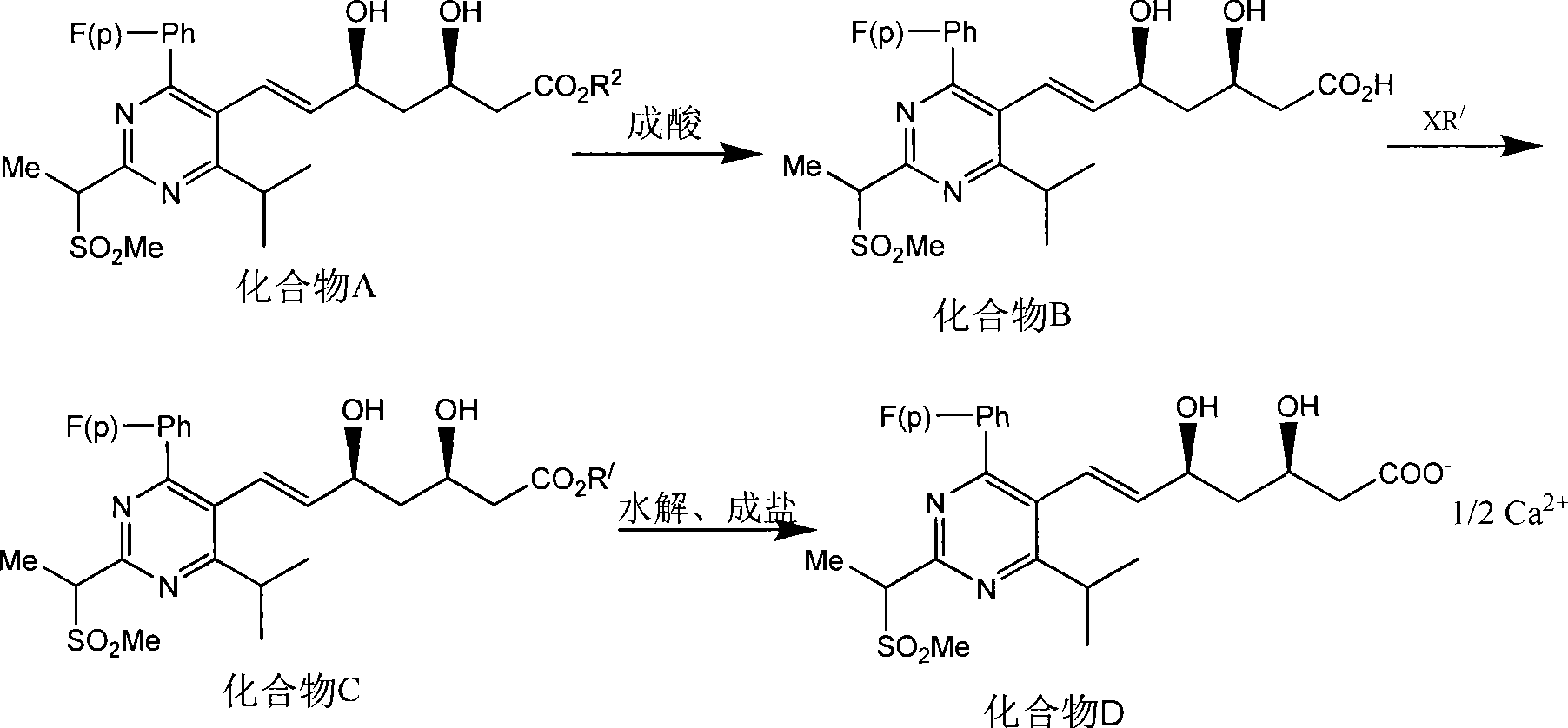
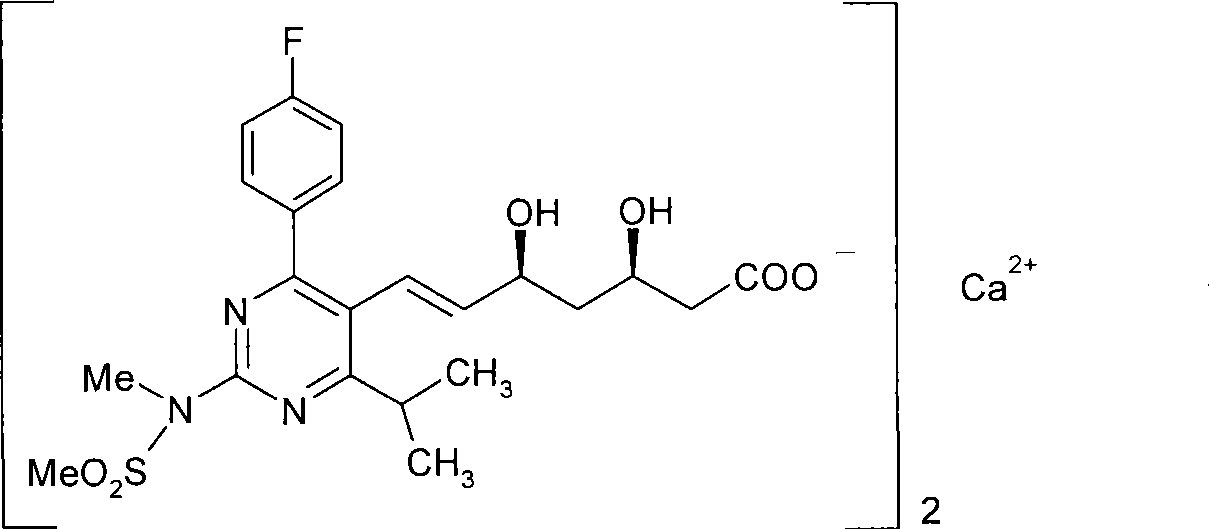
Method for synthesizing rosuvastatin intermediate and rosuvastatin
A technology of rosuvastatin calcium and a synthesis method, applied in the direction of organic chemistry and the like, can solve the problems of low reaction temperature and complexity, and achieve the effects of high purity, low equipment requirements, and simple process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] The synthetic method of compound 1:
[0059] Add 2.5L methanol, 0.8kg (6.45mol) p-fluorobenzaldehyde, 0.85kg (5.90mol) methyl isobutyryl acetate, 1kg (16.67mol) urea, appropriate amount of cuprous chloride, 2.3L1mol / L of sulfuric acid, heated to reflux (internal temperature 65-68°C) for 10 hours, cooled to 0°C in an ice-water bath, continued to stir for 4 hours, then filtered with suction, rinsed the obtained solid with methanol, and dried it in a drying oven at 60°C to obtain off-white Crystal 1.594kg, yield 92.6%. The purity of Compound 1 was determined to be 95% by HPLC.
Embodiment 2
[0061] The synthetic method of compound 1:
[0062] Add 2.5L methanol, 0.8kg (6.45mol) p-fluorobenzaldehyde, 0.85kg (5.90mol) methyl isobutyryl acetate, urea 0.885kg (14.74mol), 2.6L sulfuric acid of 1mol / L in 10L reactor, heat To reflux (internal temperature 65-68°C) for 20 hours, cool in an ice-water bath to 10°C, continue to stir for 5 hours, then filter with suction, rinse the obtained solid with methanol, and dry it at 60°C in a drying oven to obtain 1.574 kg of off-white crystals. The rate is 91.4%. The purity of Compound 1 was determined to be 95.2% by HPLC.
Embodiment 3
[0064] The synthetic method of compound 2:
[0065] Add 5L of 65-68% nitric acid and an appropriate amount of sodium nitrite in a 10L reactor, add 1.5kg (5.14mol) of compound 1 at 20°C when the temperature is controlled, and continue to react at 20-25°C for 1h. TLC detects that the reaction is complete. Add 3L of water to the reaction solution, add 30% sodium hydroxide solution dropwise at a temperature of 20°C to adjust the pH of the reaction solution to 8, cool to 5°C, continue to stir for 1h and then filter with suction, wash the obtained solid with water, and use a drying oven After drying at 80°C, 1.411 kg of a light yellow solid was obtained, with a yield of 94.7%. The purity of compound 2 was determined to be 92.8% by high performance liquid chromatography.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com