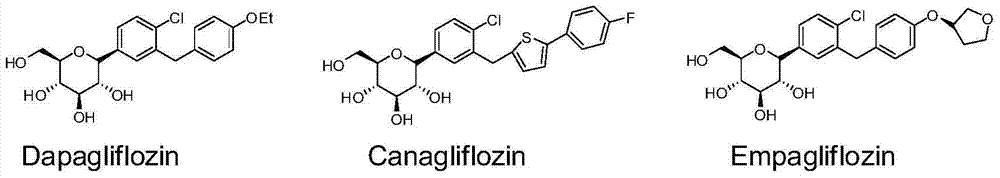
Method for synthesizing SGLT2 inhibitor drugs
A synthesis method and drug technology, applied in the field of preparation of small molecule chemical drugs, can solve the problems of difficult operation, cumbersome, high equipment requirements, etc., and achieve the effect of shortening the reaction steps, simplifying the operation method, and simple process steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
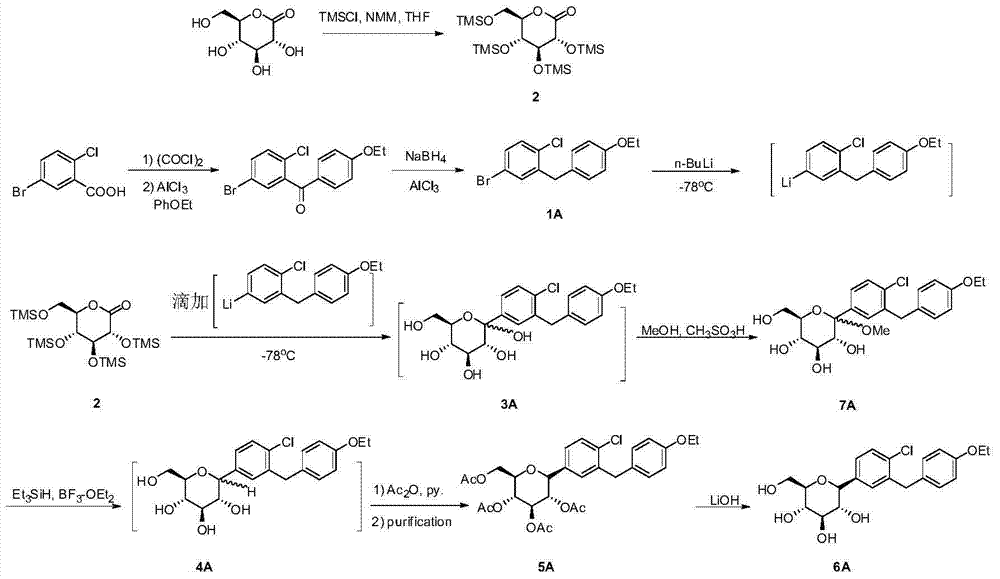
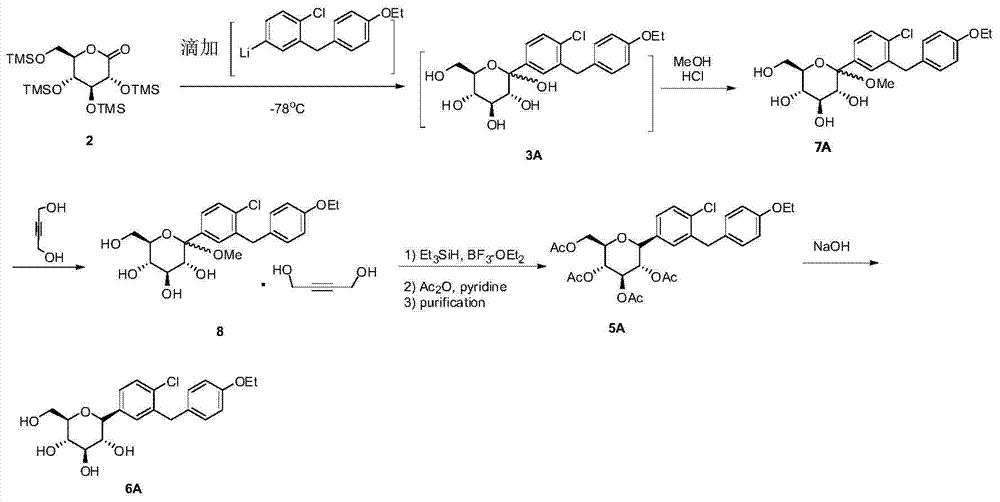
[0049] The preparation of compound (1A) and compound (2) refers to reference document US7919598 (2011).
[0050] Preparation of compound (3A)
[0051]
example 1-1
[0053] Compound (1A) (18g, 0.055mol) was dissolved in a mixed solvent of tetrahydrofuran and toluene (1:2, 150mL) in a three-neck flask protected by nitrogen, cooled to -90°C, and 2.0M n-butyllithium in hexyl Alkanes solution (40mL, 0.08mol), stirred for 30min. Then, under the protection of nitrogen, a toluene solution (50 mL) of compound (2) (28.0 g, 0.06 mol) was added dropwise. After stirring at the same temperature for 2 hours, add hydrochloric acid to adjust the pH to 4.0-6.0, separate the organic phase, extract the aqueous phase with ethyl acetate, combine the organic phases, wash with saturated brine, dry over anhydrous sodium sulfate and filter, and concentrate the filtrate to obtain 18.7 g yellow oil (80% yield, mixture of α- and β-isomers (α / β=15 / 85).
[0054] The HNMR data of the main component β-isomer are as follows:
[0055] 1 H-NMR (400MHz, CD 3 OD,400MHz,δppm): 1.34(t,J=6.8Hz,3H),3.04(m,1H),3.38(d,J=8.6Hz,1H),3.50(m,1H),3.72(t,J =9.0Hz,1H),3.76(dd,J=5.0Hz ...
example 1-2
[0057] Compound (1A) (18g, 0.055mol) was dissolved in 2-methyltetrahydrofuran and benzene mixed solvent (1:1, 120mL) in a nitrogen-protected three-neck flask, cooled to -78°C, and 2.0M n-butyl Lithium in hexane (40 mL, 0.08 mol), stirred for 1.5 hours. Then, under nitrogen protection, a benzene solution (50 mL) of compound (2) (28.0 g, 0.06 mol) was added dropwise. After stirring at the same temperature for 3 hours, add acetic acid to adjust the pH to 4.0-6.0, separate the organic phase, extract the aqueous phase with ethyl acetate, combine the organic phases, wash with saturated brine, dry over anhydrous sodium sulfate, and filter, and concentrate the filtrate to obtain 18.0 g of yellow oil (77% yield, mixture of α- and β-isomers (α / β=16 / 84).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com