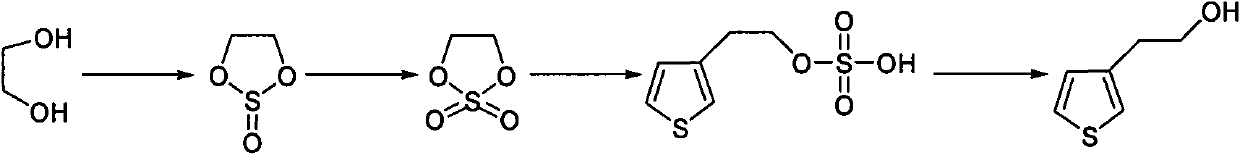
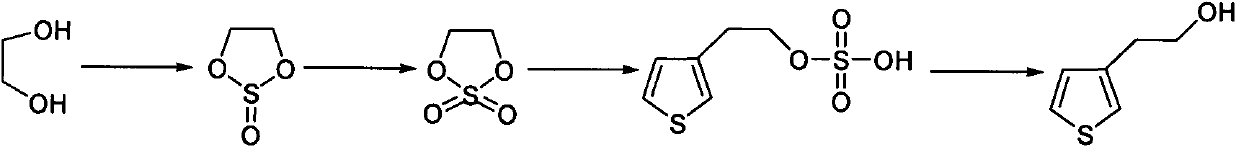
Synthetic method of thiophene-3-ethanol
A synthesis method and ethanol technology are applied in the synthesis field of thiophene-3-ethanol, can solve the problems of high price of thiophene-3-acetic acid, difficult to remove isomer impurities, unsuitable for large-scale production, etc. The effect of avoiding the generation of isomers and being easy to operate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) prepare ethylene sulfite,
[0034] Into the 1000L reactor, add 130.0kg ethylene glycol (1.0eq), 689.0kg dichloromethane (1kg / 4L), cool the system down to 30-40°C, slowly add 249.5kg thionyl chloride (1.0eq), Insulation reaction. After the reaction is complete, add sodium carbonate solution to adjust the pH of the system to 8-9, separate liquids, and extract to obtain the organic phase. Contains 223.2kg of product. Yield 98.5%, gas chromatography purity (GC): 98.42%.
[0035] (2) preparation of 1,3,2-dioxazolethiophene-2,2-dioxide,
[0036] Into the 3000L titanium steel reactor, add 113.1kg (1.0eq) system and 450.0kg dichloromethane (1kg / 3L), in the system, add dropwise 789.1kg 4.3% sodium bicarbonate aqueous solution (1kg / 0.3kg) and 1.6% 20.7kg ruthenium trichloride aqueous solution ( 0.0012eq), temperature control 0~10°C, add 119.9kg sodium hypochlorite (1.2eq) aqueous solution dropwise, keep warm for reaction, after the system is detected to be non-oxi...
Embodiment 2
[0043] (1) prepare ethylene sulfite,
[0044] Add 100.0kg of ethylene glycol (1.0eq) and 770kg of chlorobenzene (1kg / 7L) to a 1000L reactor, cool the system down to 30-40°C, slowly add 383.0kg of thionyl chloride (2.0eq), and keep warm for the reaction . After the reaction is complete, add sodium hydroxide solution to adjust the pH of the system to 8-9, separate liquids, and extract to obtain the organic phase. Contains 168.1kg of product. Yield 96.5%, gas chromatography purity (GC): 98.1%.
[0045] (2) preparation of 1,3,2-dioxazolethiophene-2,2-dioxide,
[0046] Into the 3000L titanium steel reactor, add 84kg (1.0eq) system and 447kg dichloromethane (1kg / 3L), in the system, drop 5% 840kg sodium bicarbonate aqueous solution (1kg / 0.5kg) and 2% 17.4kg ruthenium trichloride aqueous solution (0.0018eq ), control the temperature at 0-10°C, add 74.2kg of sodium hypochlorite (1.0eq) aqueous solution dropwise, and keep warm for reaction. After the system is tested to be n...
Embodiment 3
[0053] (1) prepare ethylene sulfite,
[0054] Add 65.0kg of ethylene glycol (1.0eq) and 215.3kg of dichloromethane (1kg / 2.5L) into a 1000L reactor, cool the system down to 30-40°C, and slowly add 62.3kg of thionyl chloride (0.5eq) , insulation reaction. After the reaction is complete, add potassium carbonate solution to adjust the pH of the system to 8-9, separate liquids, and extract to obtain the organic phase. Contains 106.9kg of product. Yield 94.3%, gas chromatography purity (GC): 98.1%.
[0055] (2) preparation of 1,3,2-dioxazolethiophene-2,2-dioxide,
[0056] To the 3000L titanium steel reactor, add 53.4kg (1.0eq) system and 411.2kg chlorobenzene (1kg / 7L), add dropwise 120kg sodium bicarbonate aqueous solution (1kg / 0.1kg) of 4.5% and 1.5% 14.6kg ruthenium trichloride aqueous solution (0.0018eq ), control the temperature at 0-10°C, add 129.3kg of sodium hypochlorite (3eq) aqueous solution dropwise, and keep the temperature for reaction. After the system is de...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com