Method for synthesizing lithium sulfide
A lithium sulfide and lithium iodide technology, applied in the directions of alkali metal sulfide/polysulfide, lithium storage battery, electrochemical generator, etc., can solve the problems of low solvent selectivity, harsh preparation conditions, complex synthesis process, etc. Simple and easy process steps and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Step 1: Weigh 30.842 g of biphenyl and dissolve it in a corresponding volume of anhydrous ethylene glycol dimethyl ether solvent at a concentration of 1 mol / L. After the biphenyl is completely dissolved, add 1.3882 g of lithium metal with a length of 1 cm and a width of 1 mm Silk, let it stand until it is completely dissolved;
[0032]Step 2: Weigh 3.206 g of elemental sulfur into the above lithiated solution, and stir at room temperature for 6 h;
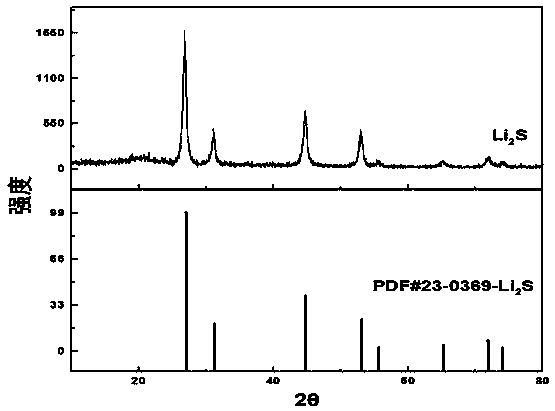
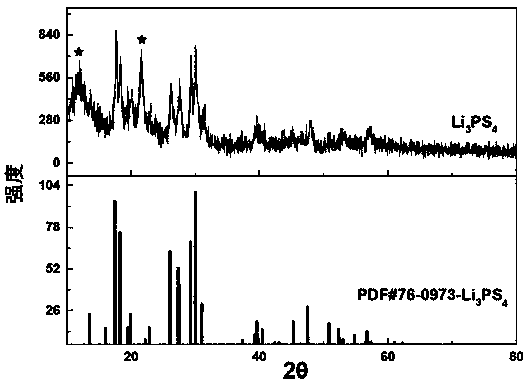
[0033] Step 3: Take out the product, separate the precipitate and supernatant with a centrifuge, the speed is 8000 r / min, and the centrifugation time is 20min, and the supernatant is recovered for reuse; the precipitate is centrifuged with anhydrous ethylene glycol xylene solvent Wash 3 times at 6000r / min, centrifuge for 10 min; dry in a vacuum oven at 100 °C for 16 h, and heat-treat at 200 °C for 8 h under the protection of argon to remove residual solvent to obtain lithium sulfide material. figure 1 is the XRD pattern of ...
Embodiment 2
[0035] Step 1: Weigh 25.636 g of naphthalene and dissolve it in a corresponding volume of anhydrous tetrahydrofuran solvent at a concentration of 0.1 mol / L. After the biphenyl is completely dissolved, add 1.3882 g of lithium metal with a length of 2 mm, a width of 2 mm, and a thickness of 0.5 mm. Let it stand until it dissolves completely;
[0036] Step 2: Weigh 3.206 g of elemental sulfur into the above lithiated solution, and stir at room temperature for 4 h;
[0037] Step 3: Take out the product, separate the precipitate and supernatant with a centrifuge at a speed of 8000 r / min, and centrifuge for 20 minutes, recover the supernatant for reuse, and wash the precipitate with anhydrous tetrahydrofuran solvent for 3 times, The rotational speed was 6000 r / min, and the centrifugation time was 10 min; the samples were dried on a heating platform at 60 °C for 24 h under the protection of argon, and then heat-treated in a vacuum oven at 180 °C for 12 h to remove residual solvents t...
Embodiment 3
[0039] Step 1: Weigh 26.7692 g of lithium iodide and dissolve it in a corresponding volume of ultra-dry acetonitrile solvent at a concentration of 10 mol / L;
[0040] Step 2: Weigh 3.206 g of elemental sulfur into the above lithiated solution, and stir at room temperature for 8 h;
[0041] Step 3: Take out the product, separate the precipitate and supernatant with a centrifuge at a speed of 8000 r / min, and the centrifugation time is 20 min. The precipitate is centrifuged and washed 3 times with an ultra-dry acetonitrile solvent at a speed of 6000 r / min, and the centrifugation time is 10 min; followed by drying in a vacuum oven at 140 °C for 12 h, and heat treatment at 320 °C under argon protection for 6 h to remove residual solvents to obtain lithium sulfide materials.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com