Organic solar cell acceptor material using diindeno bithiophene as core, preparation method and applications thereof
A technology for solar cells and acceptor materials, applied in the field of organic solar cell acceptor materials and their preparation, can solve the problems of single chemical structure, unfavorable acceptor stacking arrangement, limited regulation of material band gap and spectral absorption, etc. Simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
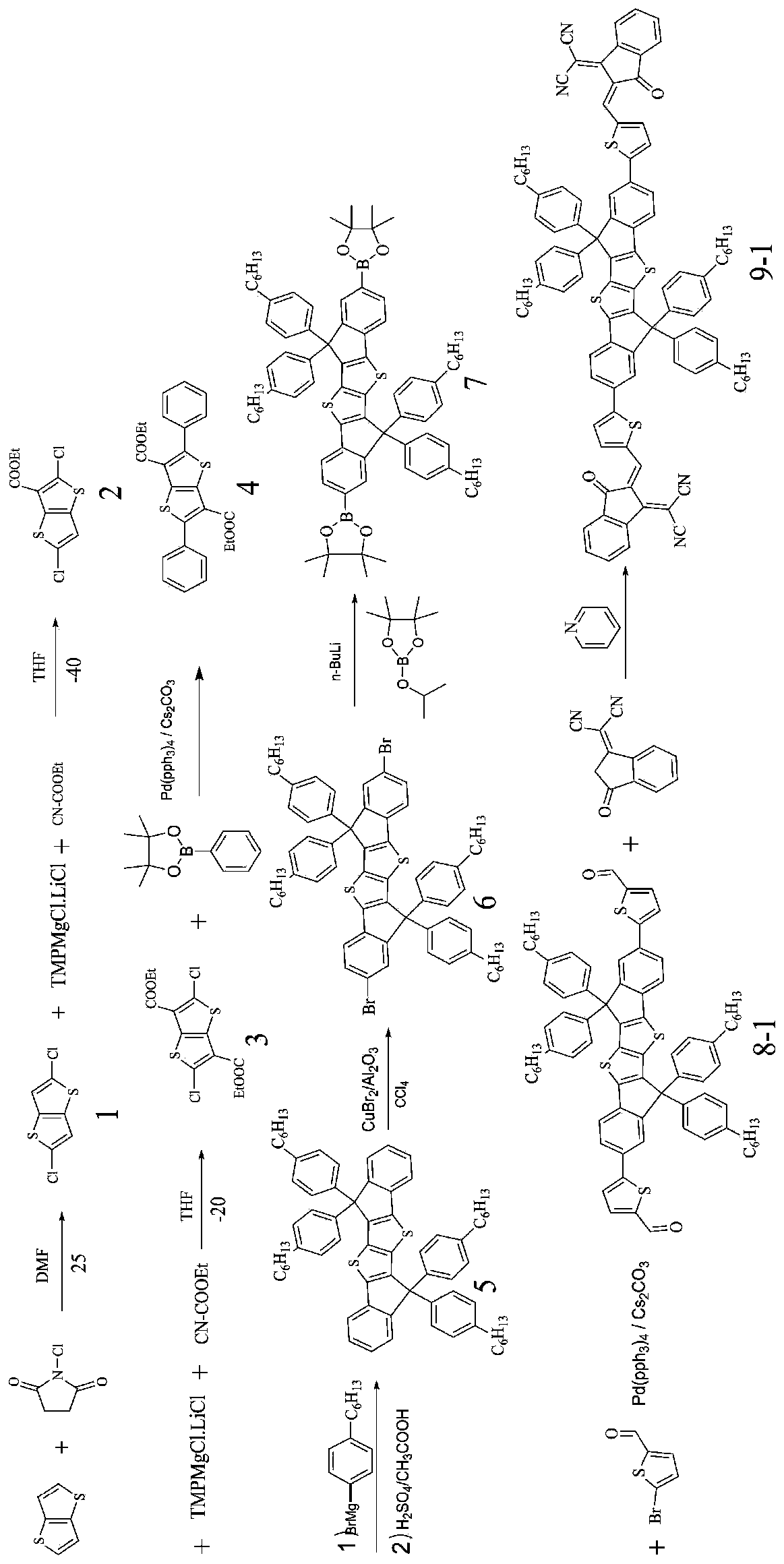
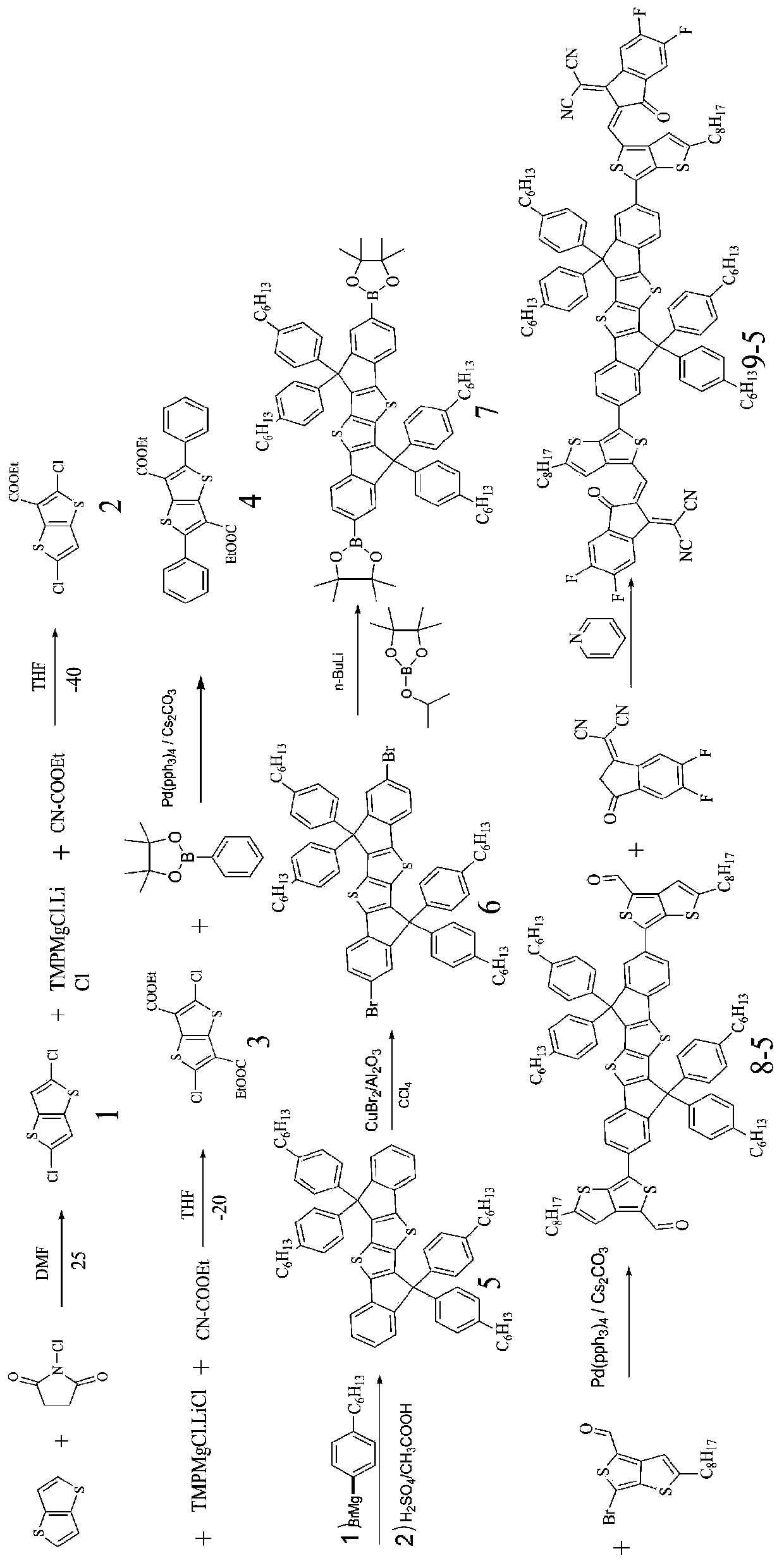
[0057] The structural formula of the organic solar cell acceptor material (SMOPV-1) in which the conjugated fused ring small molecule is the nucleus of this embodiment is:
[0058]
[0059] The preparation method of SMOPV-1 comprises the following steps (1)~(8):
[0060] (1) Synthesis of compound 1:
[0061]
[0062] Take a single-necked round-bottom flask, add N-chlorosuccinimide to the N,N-dimethylformamide solution of thiophene and stir at room temperature for several hours, monitor by TLC, add water to quench the reaction, and The liquid was transferred to a separatory funnel and extracted with ether, the organic phase was dried with anhydrous sodium sulfate, spin-dried and passed through the column with petroleum ether eluent to obtain a white solid, namely the double-sided chlorinated thiophene product 1; wherein, N-chloro The ratio of succinimide, thiophene and N,N-dimethylformamide is 10-20mmol:20-50mmol:40-100mL; the yield is 90.4%; the structural characterizat...
Embodiment 2
[0086] The structural formula of the organic solar cell acceptor material (SMOPV-2) in which the conjugated fused ring small molecule is the nucleus of this embodiment is:
[0087]
[0088] The preparation method of SMOPV-2 comprises following steps (1)~(8):
[0089] Wherein, steps (1) to (7) in the preparation method of SMOPV-2 are the same as the preparation steps (1) to (7) in Example 1;
[0090] (8) Synthesis of compound 9-2:
[0091]
[0092] Take a double-necked round-bottom flask, dissolve compound 8-1 in chloroform, and carry out Knoevenagel condensation reaction with difluoromalononitrile indanone pulling electron unit under weakly alkaline conditions. After the reaction is monitored by TLC, the reaction solution is directly Pass through the column with petroleum ether / dichloromethane eluent to obtain the finally described organic solar cell with bisindenobithiophene as the core; wherein, the proportion of compound 8-1, electron-drawing unit, pyridine, and chloro...
Embodiment 3
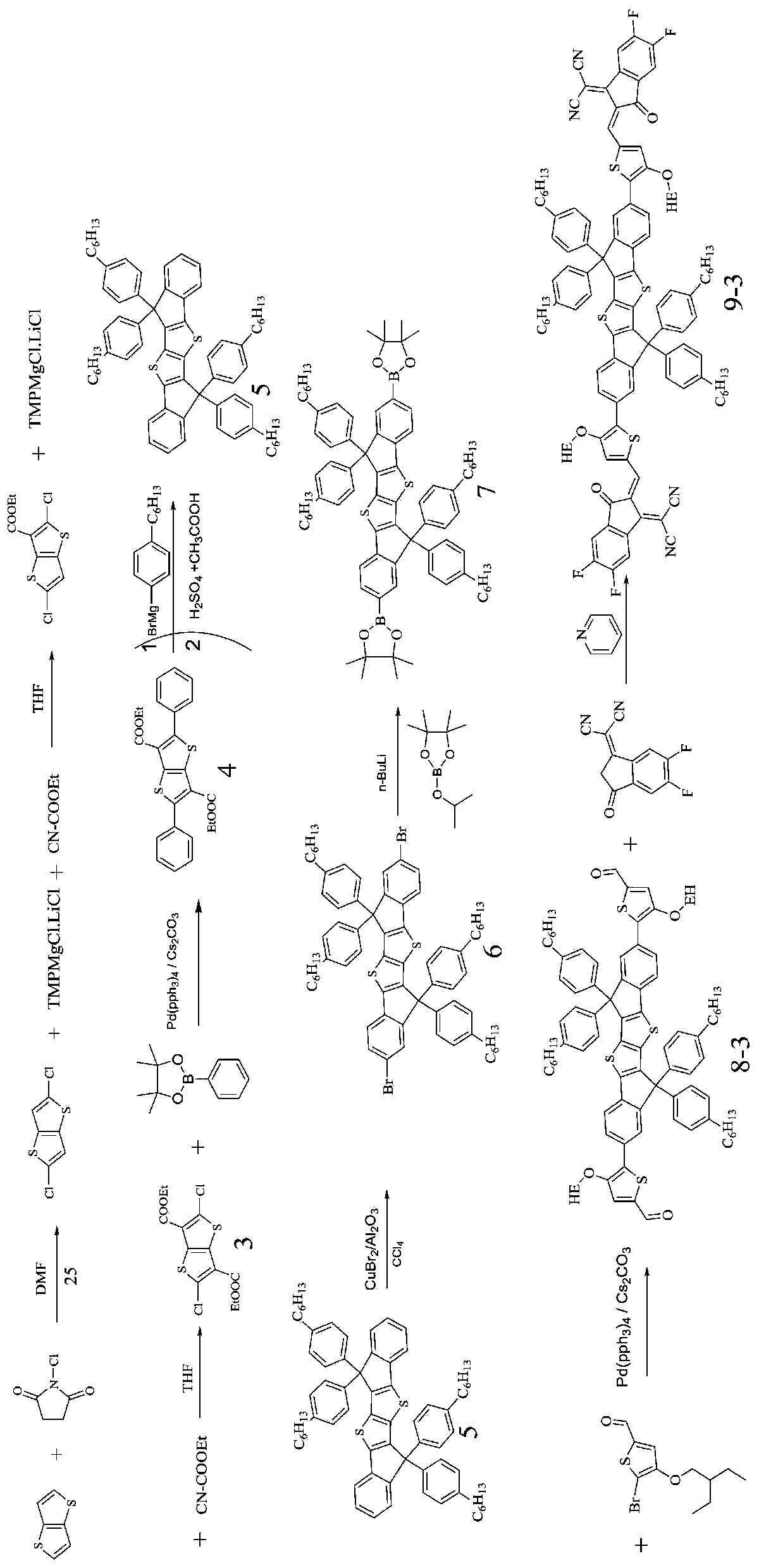
[0094] The structural formula of the organic solar cell acceptor material (SMOPV-3) in which the conjugated fused ring small molecule is the nucleus of this embodiment is:
[0095]
[0096] The preparation method of SMOPV-3 comprises the following steps (1)~(8):
[0097] Wherein, steps (1) to (6) in the preparation method of SMOPV-3 are the same as the preparation steps (1) to (6) in Example 1;
[0098] (7) Synthesis of compound 8-2:
[0099]
[0100] Take a double-necked round-bottom flask, anhydrous and anaerobic treatment, add the product 7, the π electron linking unit is 5-bromo-4-((2-ethylhexyl)oxy)thiophene-2-carbaldehyde, tetratriphenyl Phosphine palladium catalyst, cesium carbonate, replace the gas 3 times, add toluene under the protection of nitrogen to carry out Suzuki coupling reaction, heat the reaction solution to reflux reaction overnight, TLC monitoring after the reaction is completed, cool to room temperature, add water to quench, and transfer the reacti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com