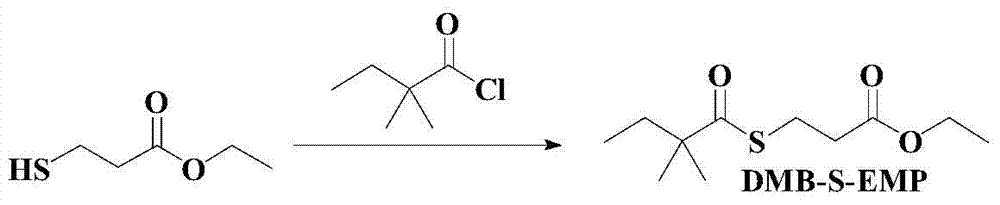
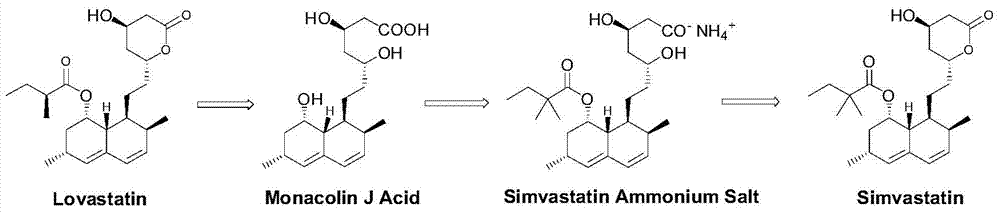
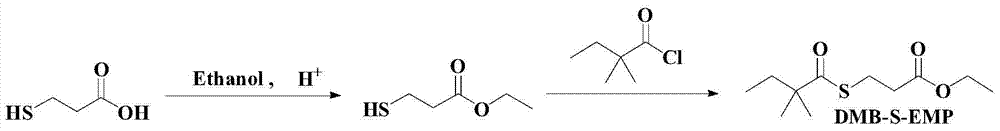
Preparation method of simvastatin side chain acyl donor
A technology of simvastatin and acyl donor, applied in the field of medicine, can solve the problems of large amount of solvent, environmental pollution and high cost of raw materials, and achieves a simple and easy synthesis method, cheap and easy-to-obtain synthetic raw materials, and strong product competitiveness. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1: Preparation method of simvastatin acyl donor (two-step method)
[0047] Step 1: Synthesis of ethyl 3-mercaptopropionate (see attached image 3 ):
[0048] In a 50mL three-neck flask, N 2 Under protection, add 20mL of absolute ethanol, 1mL of concentrated sulfuric acid, add 2 grams of anhydrous sodium sulfate, 2 grams of 3-mercaptopropionic acid, GC to track the reaction progress, react at 80 degrees for 12 hours, the raw material 3-mercaptopropionic acid is completely converted, add into 10 mL of ice water and extracted three times with dichloromethane, 10 mL each time. The dichloromethane layer of the organic phase was washed twice with saturated sodium bicarbonate solution, 10 mL each time. The dichloromethane layer of the organic phase was collected, dried over anhydrous magnesium sulfate, filtered, and the solvent was removed by rotary evaporation to obtain 2.3 g of a colorless oily product with a yield of 90.9%. ( 1 See attached for H NMR figure 1 )...
Embodiment 2
[0051] Example 2: Preparation method of simvastatin acyl donor (two-step method)
[0052] Step 1: its reaction formula is with embodiment 1
[0053] In a 250mL three-necked flask, under the protection of N2, add 100mL of absolute ethanol, 3mL of concentrated sulfuric acid, 4 grams of anhydrous magnesium sulfate, 20 grams of 3-mercaptopropionic acid. The acid conversion was complete, and the ethanol was distilled off under reduced pressure, leaving 20.5 g of colorless oil as the remaining product, with a yield of 81%. used directly in the next reaction.
[0054] Step 2: its reaction formula is with embodiment 1
[0055] Add 100mL of dichloromethane to dilute the remaining product, cool to zero, add 30 grams of sodium carbonate, N 2 For protection, 22.55 g of 2,2-dimethylbutyryl chloride was added dropwise within 10 minutes, and the reaction was carried out at zero temperature for 3 hours after the drop was completed. Naturally, the temperature was raised to about 25 degrees...
Embodiment 3
[0056] Example 3: The preparation method of simvastatin acyl donor (one-step method) (see attached Figure 5 ):
[0057] In a 250mL three-neck flask, add 100mL absolute ethanol, 3mL concentrated sulfuric acid, N 2 For protection, add 4 grams of anhydrous magnesium sulfate, 20 grams of 3-mercaptopropionic acid, GC to detect the reaction process, react at 80 degrees for 12 hours, the raw material 3-mercaptopropionic acid is completely converted, add it to 500mL ice water, and extract it with dichloromethane three times. 80mL each time. The dichloromethane layer of the organic phase was washed twice with saturated sodium bicarbonate solution, 50 mL each time. The dichloromethane layer was collected and dried over anhydrous magnesium sulfate.
[0058] Filter, the filtrate is cooled to zero degree, add 30 grams of sodium carbonate, N 2 For protection, 25.3 g of 2,2-dimethylbutyryl chloride was added dropwise within 10 minutes, and the reaction was carried out at zero temperatur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com