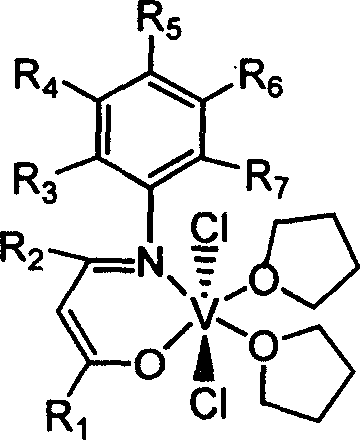
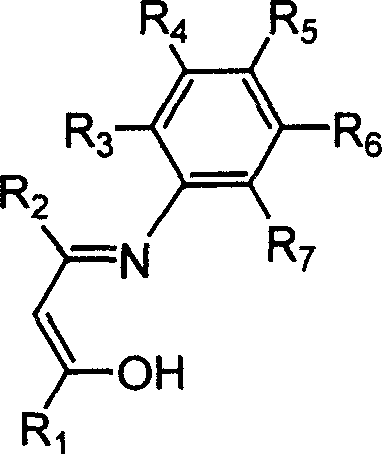
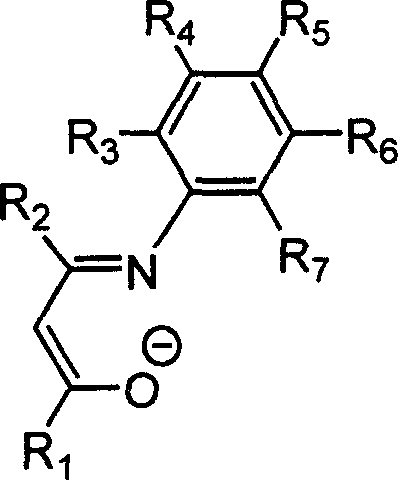
Beta-diketo mono imine vanadium olefinic polymerization catalyst, and its preparing method and use
A diketone monoimine vanadium olefin and polymerization catalyst technology, which is applied in the field of β-diketone monoimine vanadium olefin polymerization catalyst and preparation, and can solve the problems of low catalytic activity, easy deactivation, poor high temperature resistance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Add 4.00 g of acetylacetone, equivalent to 40 mmol, 4.85 g of 2,6-dimethylaniline, equivalent to 40 mmol, 15 mL of methanol, and 1 mL of formic acid into a dry 100 mL reaction flask, and heat to reflux for 24 hours. The solvent methanol was distilled off with a rotary evaporator, and petroleum ether containing 1% ethyl acetate was used as eluent, and the residue was subjected to column chromatography to obtain 4.15 g of white solid Schiff's base, with a yield of 51%. According to mass spectrometry, the molecular ion peak m / e is 203. Elemental analysis found values: C, 76.92%; H, 8.39%; N, 6.85%; theoretical value (C13H17NO): C, 76.81%; H, 8.43%; N, 6.89%.
[0035] Under a nitrogen atmosphere, add 0.406 g of the above-obtained Schiffer’s base (equivalent to 2.0 mmol) and 20 mL of anhydrous tetrahydrofuran into a dry 100 mL reaction flask, stir at room temperature for 10 min, and cool to -78°C. Within 5 minutes, 1.375 mL of a 1.60 mol / L n-butyllithium hexane solution, equi...
Embodiment 2
[0037] With 7.09g of 2,6-diisopropylaniline, which is equivalent to 40mmol, to replace 2,6-dimethylaniline in Example 1, the experimental operation is the same as in Example 1, and 4.77g of white solid Schiffer’s base is obtained. rate 46%. According to mass spectrometry, the molecular ion peak m / e is 259. Elemental analysis measured values: C, 78.61%; H, 9.68%; N, 5.44%; theoretical value (C17H25NO): C, 78.72%; H, 9.71%; N, 5.40%.
[0038] 0.52g of Schiff's base prepared in Example 2, equivalent to 2mmol, replaced the Schiff's base obtained in Example 1, and the experimental operation was the same as in Example 1 to obtain 0.51g of brown β-diketone monoimine vanadium olefin polymerization catalyst, Yield 49%. According to mass spectrometry, the molecular ion peak m / e is 524. Elemental analysis found values: C, 57.09%; H, 7.65%; N, 2.64%; theoretical value (C25H40Cl2NO3V): C, 57.26%; H, 7.69%; N, 2.67%.
Embodiment 3
[0040] Add 5.19g of 1-phenyl-1,3-butanedione, equivalent to 32mmol, 5.96g of aniline, equivalent to 64mmol, 15mL of methanol, and 1mL of formic acid into a dry 100mL reaction flask, heat and reflux for 36h, and cool the reaction solution. A colorless solid precipitated out. Filter, wash with cold petroleum ether for several times, and dry in vacuo to obtain 3.95 g of white solid Schiff's base with a yield of 52%. According to mass spectrometry, the molecular ion peak m / e is 237. Elemental analysis found values: C, 80.62%; H, 6.36%; N, 5.86%; theoretical value (C16H15NO): C, 80.98%; H, 6.37%; N, 5.90%.
[0041] 0.47g of Schiff's base prepared in Example 3, equivalent to 2mmol, replaced the Schiff's base obtained in Example 1, and the experimental operation was the same as in Example 1 to obtain 0.45g of brown β-diketone monoimine vanadium olefin polymerization catalyst, Yield 45%. Mass spectrometry, the molecular ion peak m / e is 502. Elemental analysis found values: C, 57.1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting temperature | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
| Melting temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com