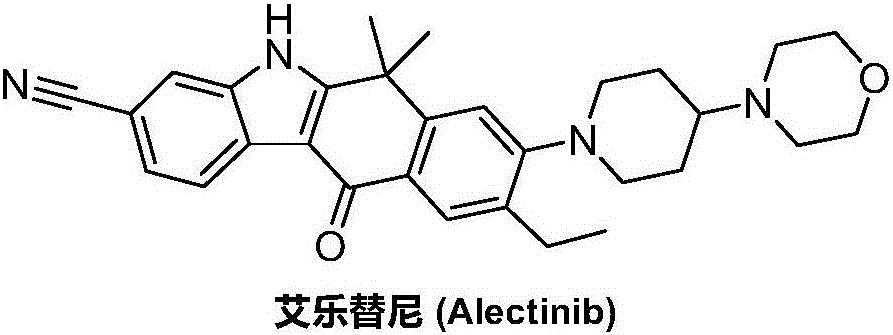
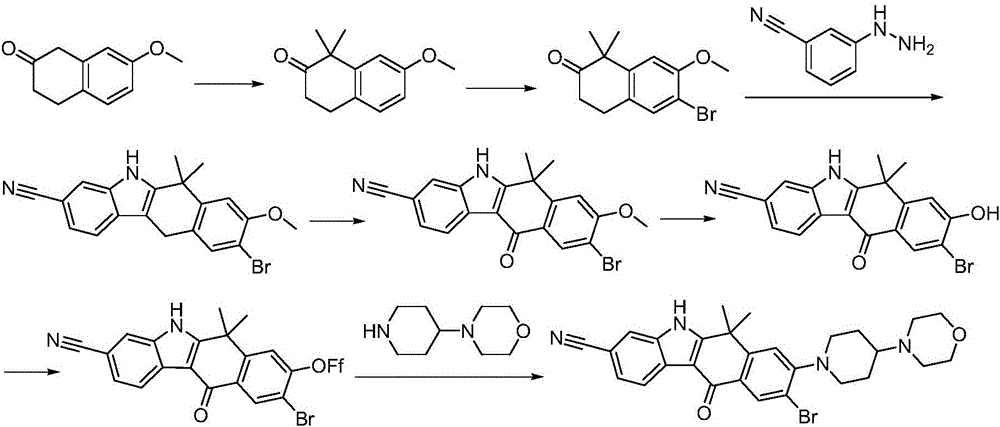
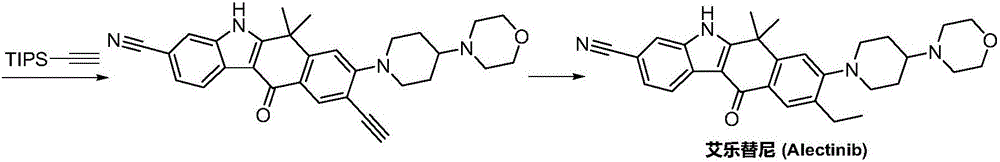
Synthesis method of Alectinib
A synthesis method and dihydrogen technology, applied in the direction of organic chemistry, etc., can solve the problems of short process flow, difficult to obtain, use a large amount of solvents, etc., and achieve the effects of reasonable technical solution, simplified operation and less impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] A) Preparation of 6-ethyl-3,4-dihydro-2-naphthone:
[0037] 6-Bromo-3,4-dihydro-2-naphthalenone (22.5g, 0.10mol) was dissolved in N,N-dimethylformamide (100mL), cooled to -78°C, and n-butyllithium was slowly added dropwise (0.12mol), the reaction mixture was stirred and reacted at -78°C for 2 hours, slowly added pinacol diboronate (38.1g, 0.15mol), the reaction mixture was stirred and reacted at -78°C for 1 hour, then naturally rose to 20°C and stirred for 10 hours, Slowly add methanol (40mL), and concentrate to dryness by rotary evaporation to obtain 3,4-dihydro-2-naphthone-6-boronic acid, add bromoethane (9.8g, 0.09mol), tetrakis(triphenylphosphine)palladium (5.8g, 5mmol), potassium carbonate (20.0g, 0.145mol), N,N-dimethylformamide (100mL) and water (60mL), the reaction mixture was heated to 95 ° C for 14 hours, TLC spot plate to determine the reaction After completion, the reaction solution was lowered to room temperature, concentrated to dryness by rotary evaporat...
Embodiment 2
[0055] A) Preparation of 6-ethyl-3,4-dihydro-2-naphthone:
[0056] 6-Bromo-3,4-dihydro-2-naphthalenone (22.5g, 0.10mol) was dissolved in N,N-dimethylacetamide (150mL), cooled to -78°C, and n-butyllithium was slowly added dropwise (0.115mol), the reaction mixture was stirred at -78°C for 2 hours, slowly added trimethyl borate (14.5g, 0.14mol), the reaction mixture was stirred at -78°C for 1.5 hours, naturally raised to 22°C and stirred for 10 hours, slowly added Methanol (40mL), concentrated to dryness by rotary evaporation to obtain 3,4-dihydro-2-naphthone-6-boronic acid, added bromoethane (10.4g, 0.095mol), [1,1'-bis(diphenyl Phosphino) ferrocene] palladium dichloride (4.8g, 6.5mmol), sodium carbonate (17.0g, 0.16mol), N,N-dimethylacetamide (150mL) and water (70mL), the reaction mixture Heat to 100°C and react for 14 hours, TLC spot plate confirms that the reaction is complete, the reaction solution is cooled to room temperature, concentrated to dryness by rotary evaporation...
Embodiment 3
[0068] A) Preparation of 6-ethyl-3,4-dihydro-2-naphthone:
[0069] 6-Bromo-3,4-dihydro-2-naphthone (22.5g, 0.10mol) was dissolved in tetrahydrofuran (120mL), cooled to -78°C, and n-butyllithium (0.115mol) was slowly added dropwise, the reaction mixture- Stir the reaction at 78°C for 2 hours, slowly add triethyl borate (23.4g, 0.16mol), stir the reaction mixture at -78°C for 2 hours, naturally rise to 25°C and stir for 10 hours, slowly add methanol (40mL), and concentrate by rotary evaporation To dryness, 3,4-dihydro-2-naphthone-6-boronic acid was obtained, bromoethane (10.9g, 0.10mol), bis(triphenylphosphine)palladium dichloride (4.2g, 6mmol), Potassium phosphate (38.2g, 0.18mol), tetrahydrofuran (120mL) and water (70mL), the reaction mixture was heated to 90 ° C for 17 hours, TLC spot plate confirmed the completion of the reaction, the reaction solution was cooled to room temperature, concentrated to dryness by rotary evaporation, added Extracted with ethyl acetate, washed w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com