Pyrazolo N-substituted dehydronorcantharidin imide derivative as well as synthesis method, activity test method and application thereof
The technology of cantharidinimide and cantharidinimide is applied in the field of pyrazolo N-substituted nordehydrocantharidinimide derivatives and their synthesis, which can solve the problem that pharmacological test results are not ideal and the inhibitory effect is lost. and other problems to achieve good anti-tumor activity
Inactive Publication Date: 2010-08-25
SHAOXING UNIVERSITY
View PDF2 Cites 34 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
There can also be a double bond between 5-C and 6-C; the 7-oxygen bridge is indispensable, which may be due to the formation of hydrogen bonds between the oxygen atom and PP1 and PP2A; 2) the reduction of the anhydride on the anhydride ring will Loss of inhibition of PP1 and PP2A
The modification of cantharidin structure 5-C or 6-C generally only introduces substituents, but the results of pharmacological tests are not ideal
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
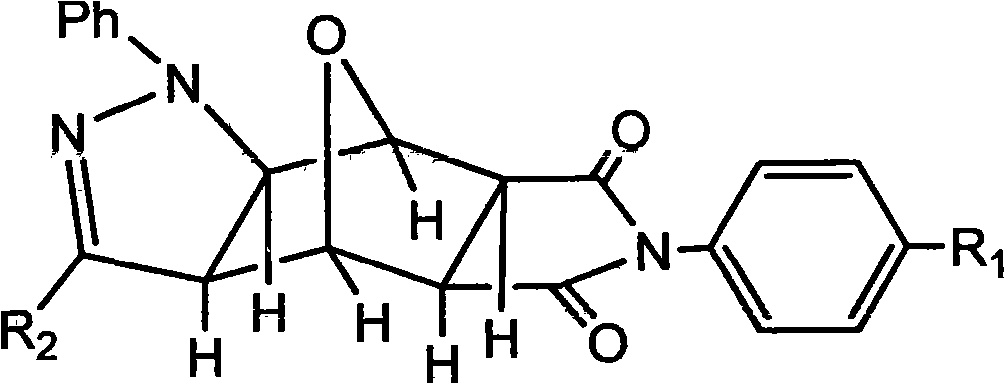
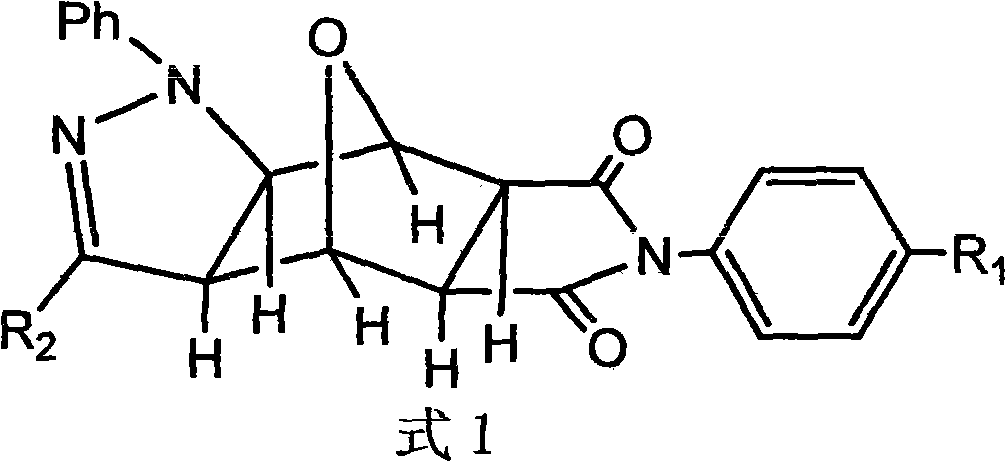
The invention discloses a pyrazolo N-substituted dehydronorcantharidin imide derivative as well as a synthesis method, an activity test method and application thereof, belonging to the field of cantharidin derivatives. The pyrazolo N-substituted dehydronorcantharidin imide derivative has a structural general formula shown as a formula 1: in the formula 1, R1 is H, C1, F, CH3, OCH3, OH or NO2; and R2 is 2-phenyl-2H-1,2,3-triazole-4-substituent or quinoxaline-2-substituent. The novel N-substituted dehydronorcantharidin imide derivative introduces five-membered heterocyclic pyrazole rings into norcantharidin substituted arylamine and has favorable anti-tumor activity.
Description
Technical field: The invention belongs to the field of cantharidin derivatives, and more specifically relates to a novel pyrazolo N-substituted nordehydrocantharidin imide derivative and its synthesis method, activity testing method and application. Background technique: Cantharidin (cantharidin) is a folk medicine in my country, and it is an active ingredient of a drug for treating malignant tumors. Modern studies have proved that it has a certain curative effect on primary liver cancer, and has the advantages of increasing white blood cells and not suppressing the immune system. Therefore, it has high medicinal research value and has attracted widespread attention. But the toxicity of cantharidin is bigger, and synthesis is very complicated, recent research shows, in norcantharidin (norcantharidin), two methyl groups of 2 and 3 positions are missing, and norcantharidin not only keeps strong antitumor activity and The unique effect of increasing white blood cells, and the ...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07D491/22C12Q1/02A61K31/4192A61K31/498A61P35/00
Inventor 邓莉平
Owner SHAOXING UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com