Dihydropyridine calcium antagonist co-crystal and preparation method and application thereof
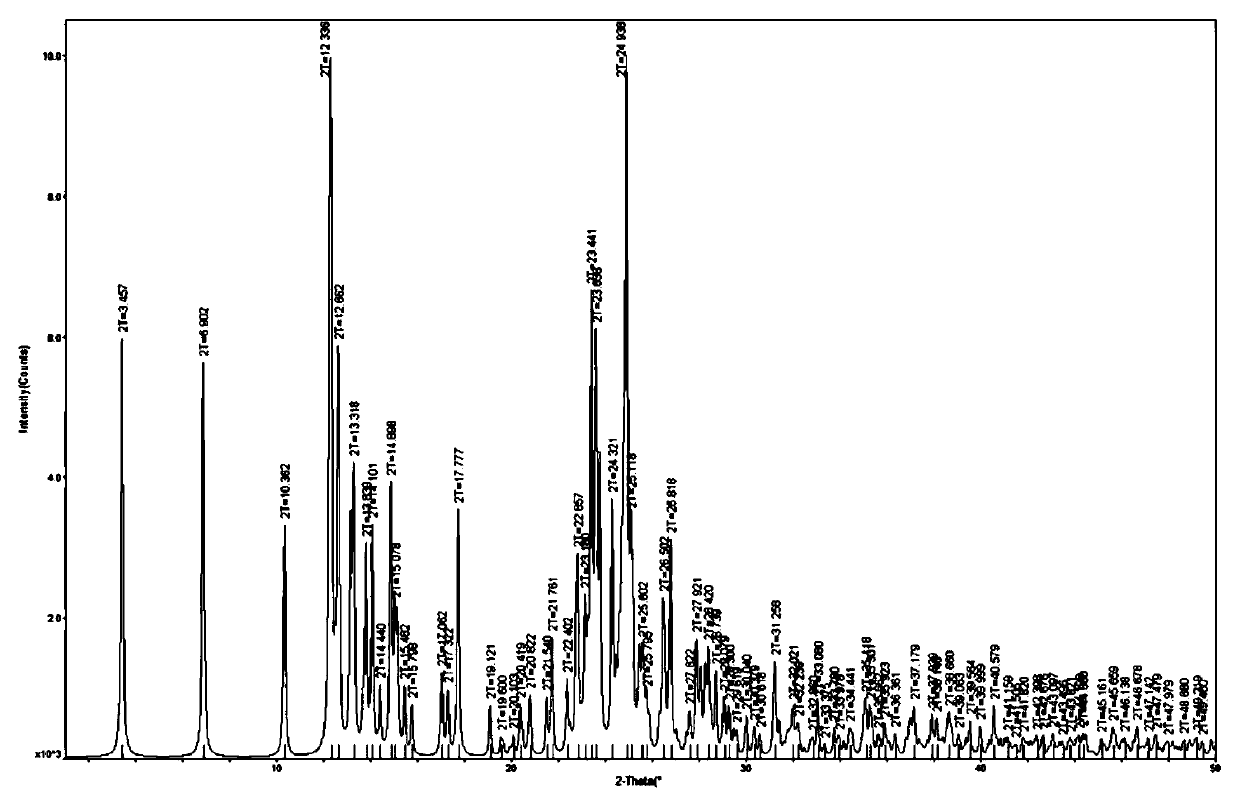
A dihydropyridine calcium antagonism and co-crystal technology, applied in the field of crystal form medicines, can solve problems such as unsatisfactory stability, unsatisfactory curative effect of levamlodipine maleate, etc. - X-ray powder diffraction data, the effect of improving solubility and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Preparation of Levoamlodipine Benzoate Crystals
[0047] 1) Weigh 2.04 grams of levoamlodipine free base, add it to 4.5 mL of aqueous solution, and stir at room temperature until completely dissolved;
[0048] 2) Prepare an aqueous solution of benzoic acid with a concentration of 1M at room temperature, and add 5 mL of the aqueous solution of benzoic acid to the solution of 1) dropwise within 30 seconds under stirring at room temperature;
[0049] 3) Transfer the above-mentioned salt-forming reaction solution to a reaction kettle, increase the temperature of the reaction solution to 50°C, keep it warm for 5 hours, and then let it stand and cool to 5°C to obtain a large number of needle-shaped or rod-shaped colorless, transparent, and uniform crystal product;
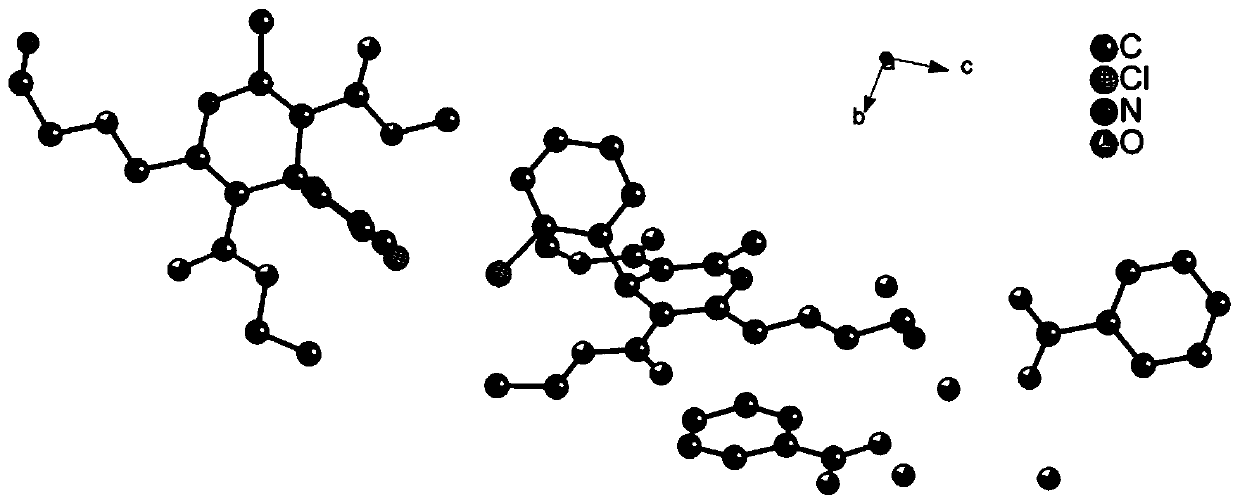
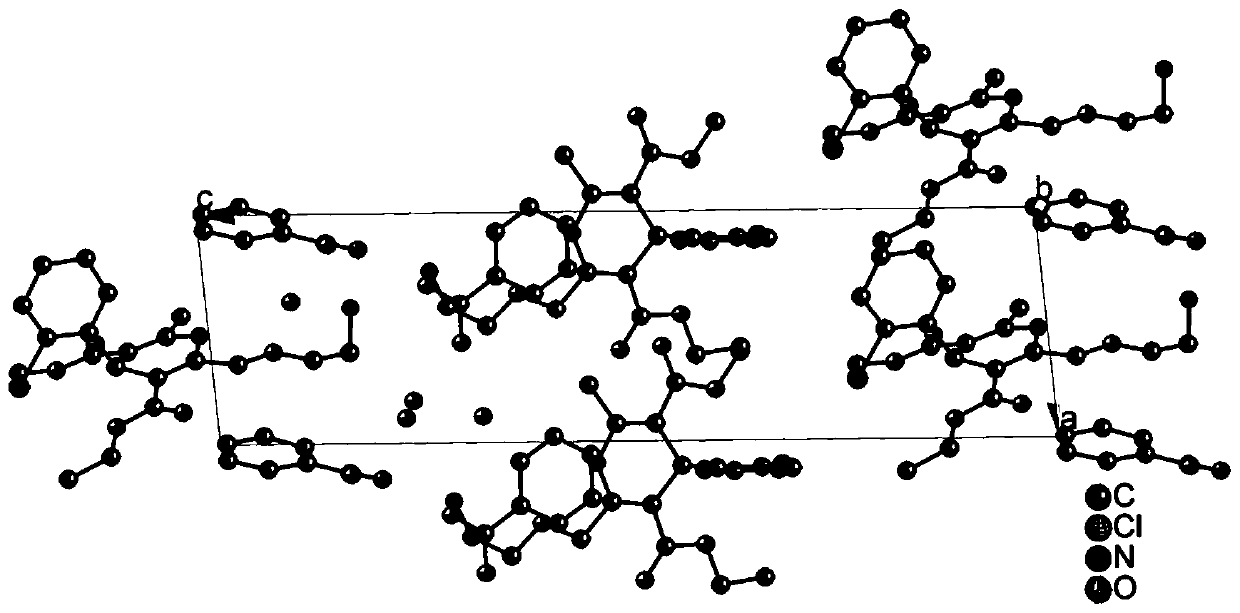
[0050] 4) Filter the obtained crystalline product under reduced pressure, wash the original crystalline solution, and wash with a water solvent at 5°C to obtain, figure 1 is a schematic diagram of the crystal st...
Embodiment 2-8
[0052] The preparation method is the same as in Example 1, and its specific process parameters are shown in Table 1.
[0053] Table 1 embodiment 2-8 process parameter
[0054]
Embodiment 9
[0056] X-ray single crystal diffraction test
[0057] Taking the levamlodipine benzoate eutectic crystal prepared in Example 1 as an example, the crystal quality and size of the selected crystal meet the requirements of the test instrument, and the X-ray single crystal diffractometer of the Apex Duo model of Bruker, Germany is used to test, and the test parameters Follow the strategy established by the instrument. The test temperature is 296K, with Mo-Kα radiation Diffraction data were collected in ω-scan mode and Lp correction was performed. Absorption correction was performed using the SADABS program. The structure is analyzed by the direct method, all non-hydrogen atoms are found by the difference Fourier method, all hydrogen atoms on carbon and nitrogen are obtained by theoretical hydrogenation, and the structure is corrected by the least square method. All parsing processes are completed using the SHELXTL program package.
[0058] In the above measurement, the crysta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com