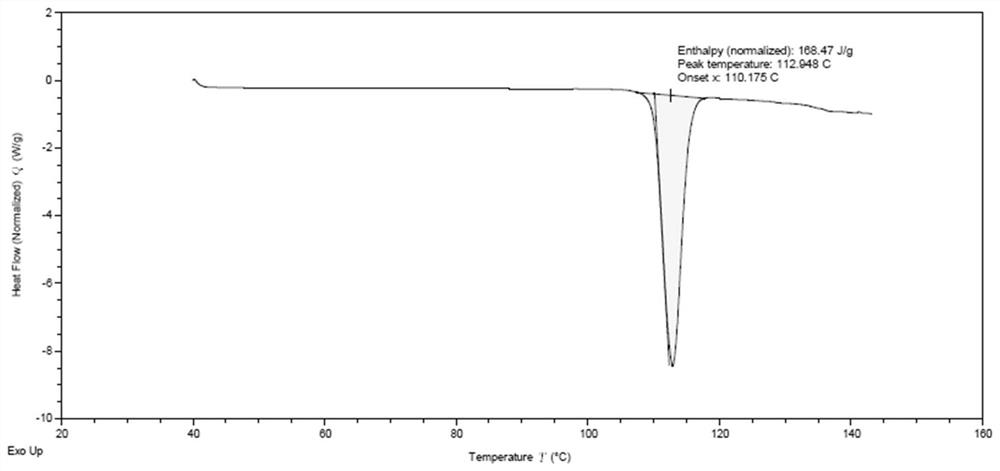
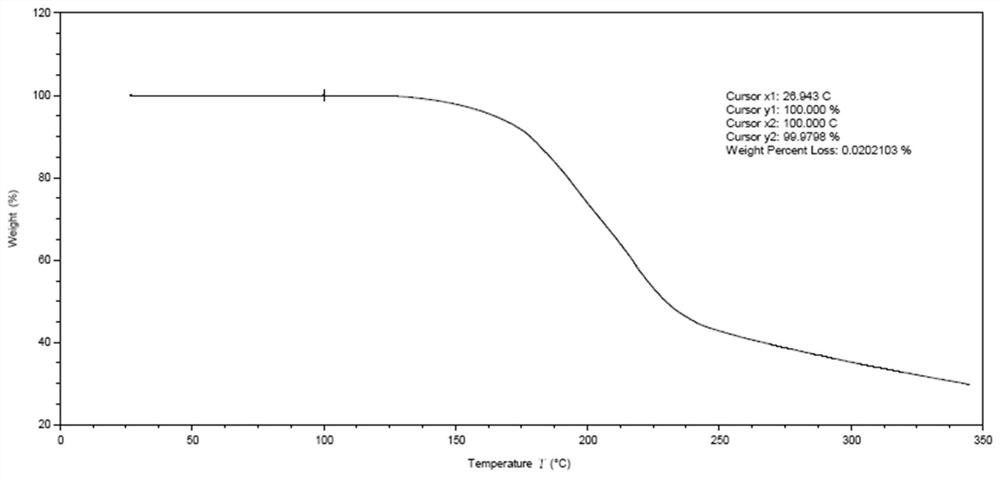
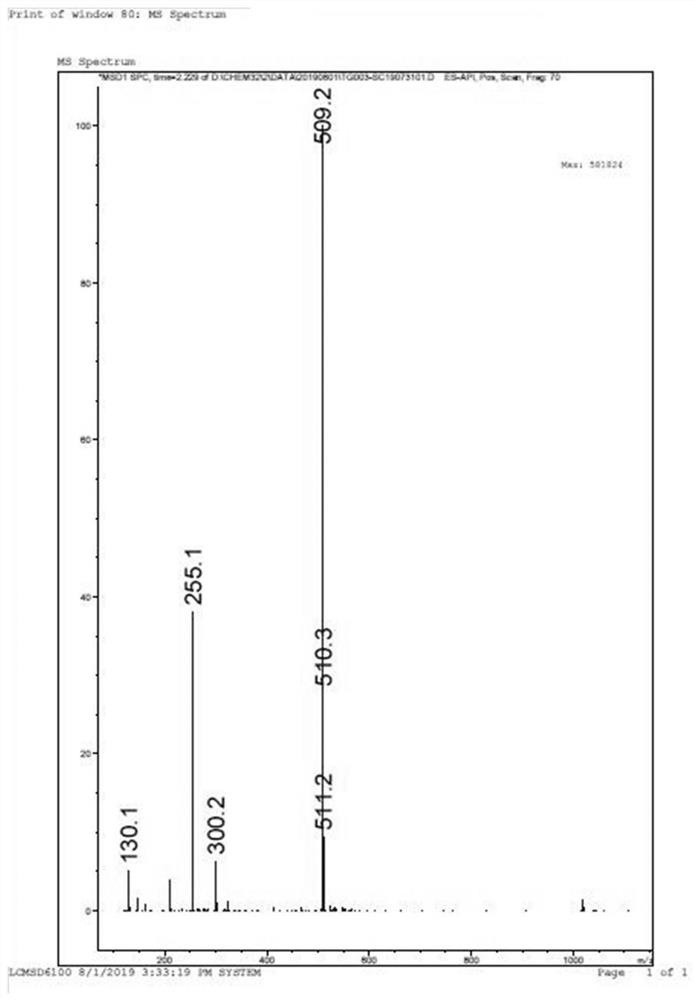
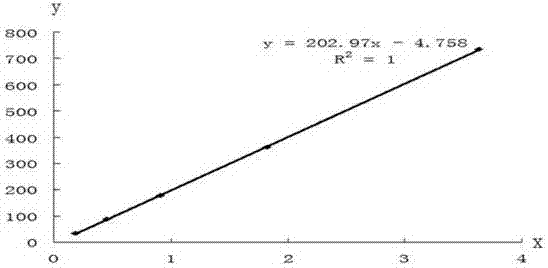
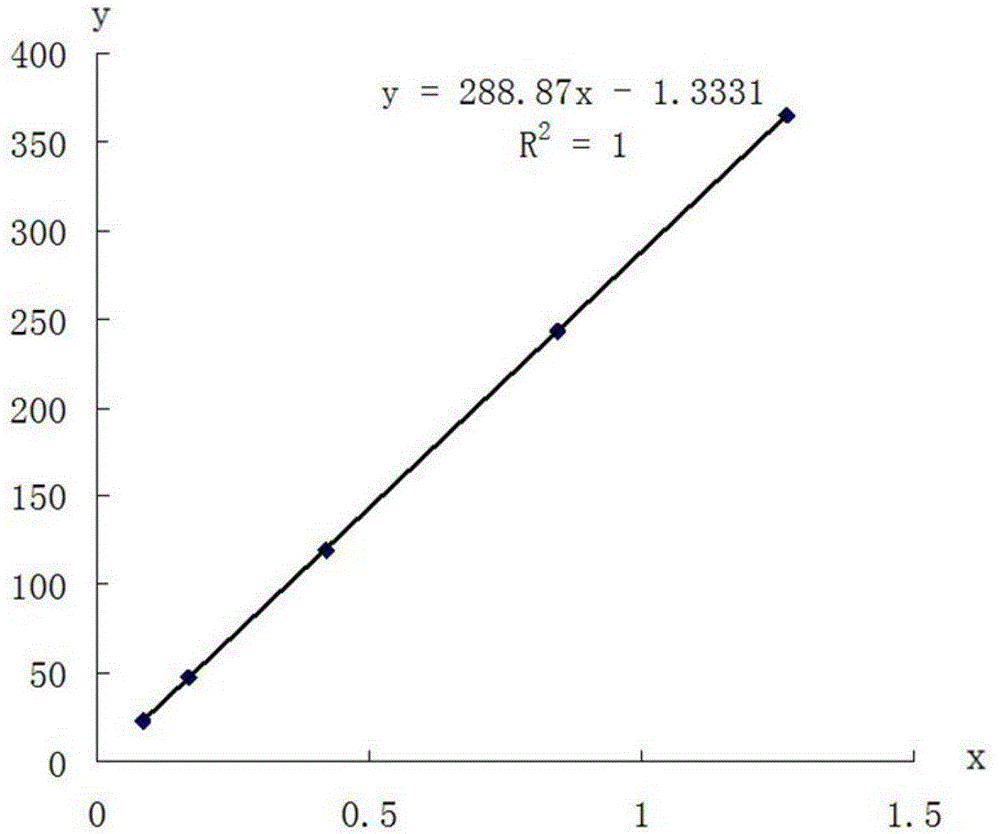
Patents
Literature
38results about How to "Applicable quality control" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
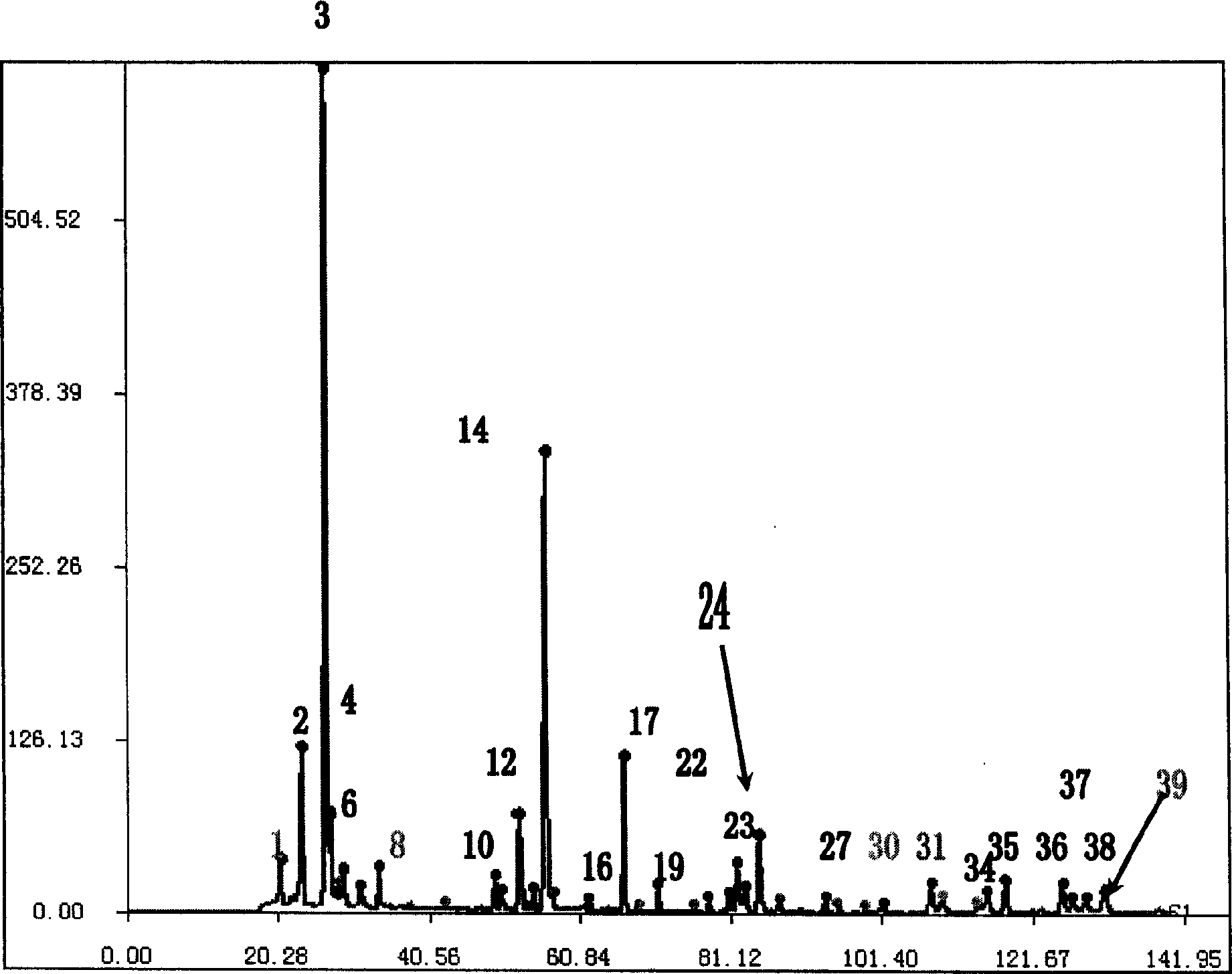
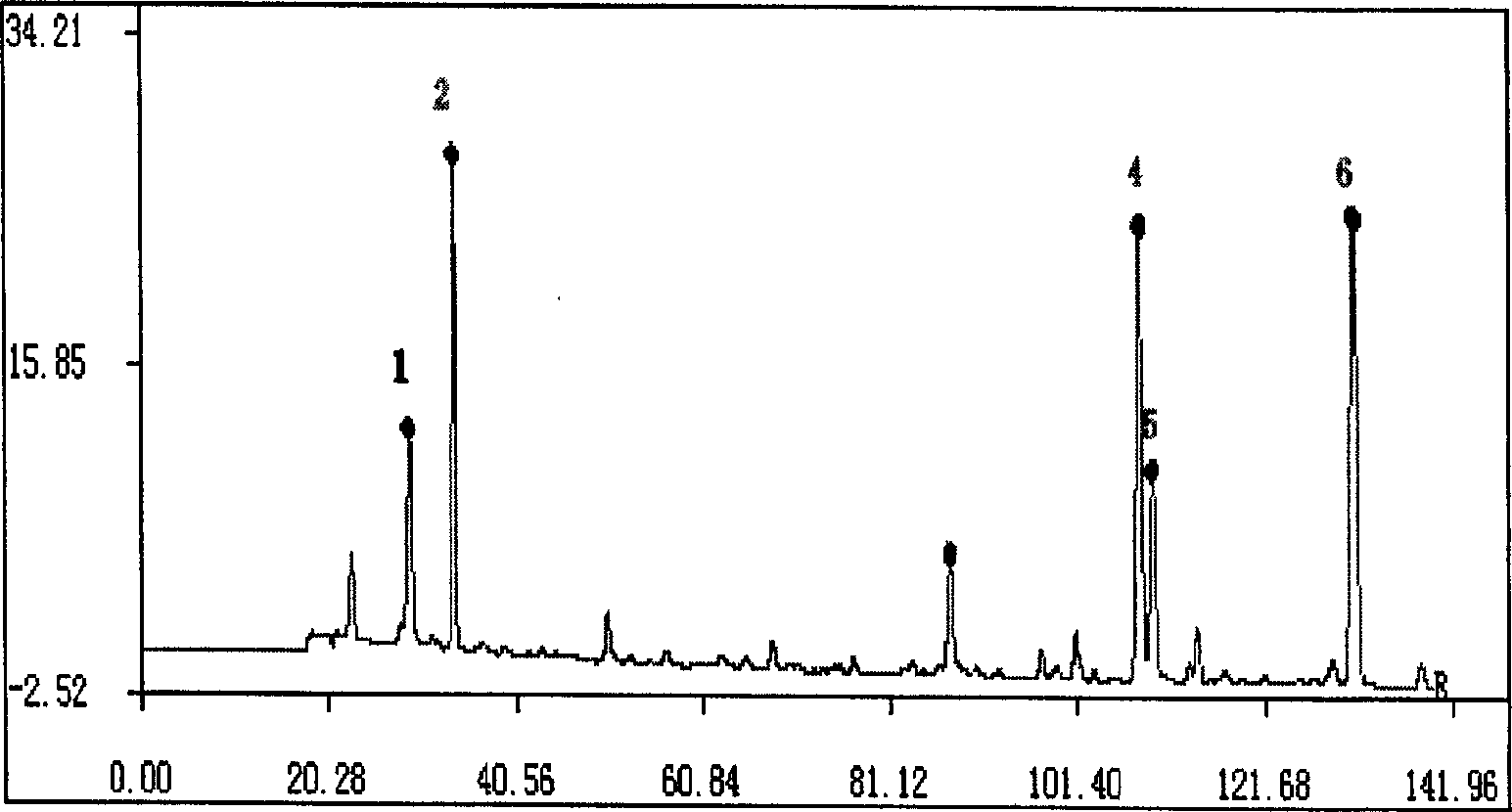
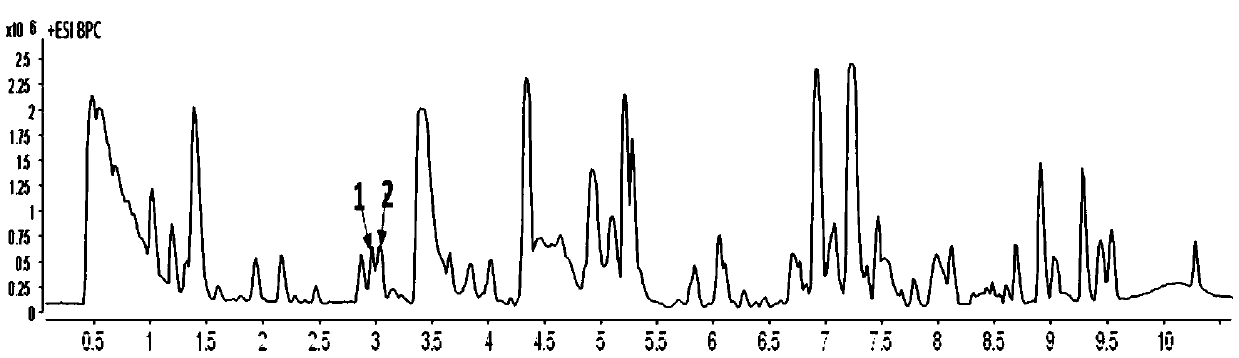
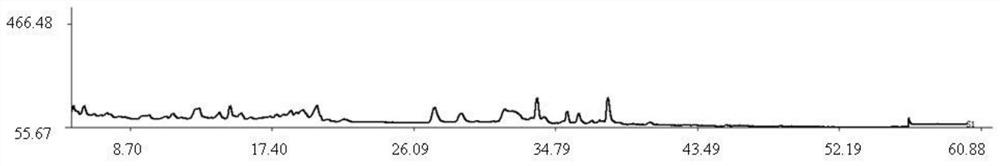
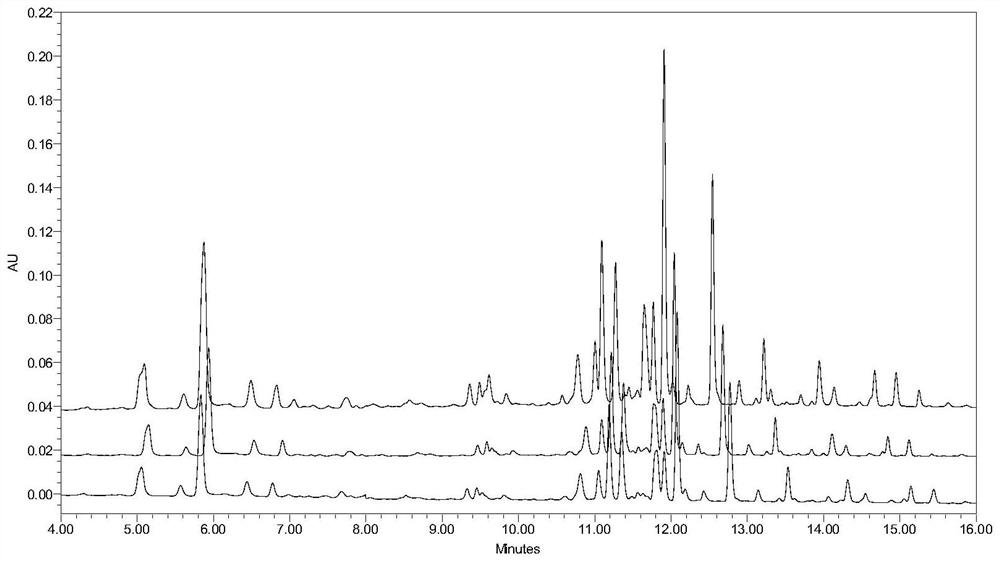
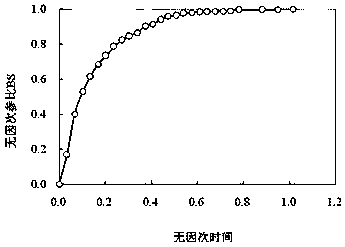
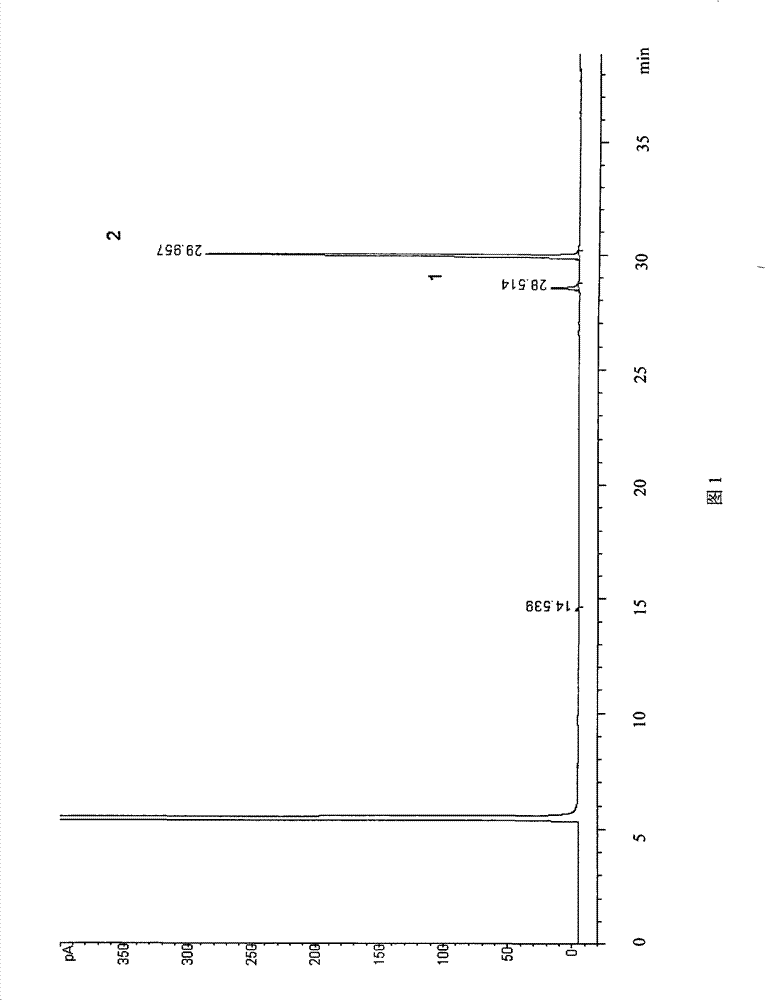
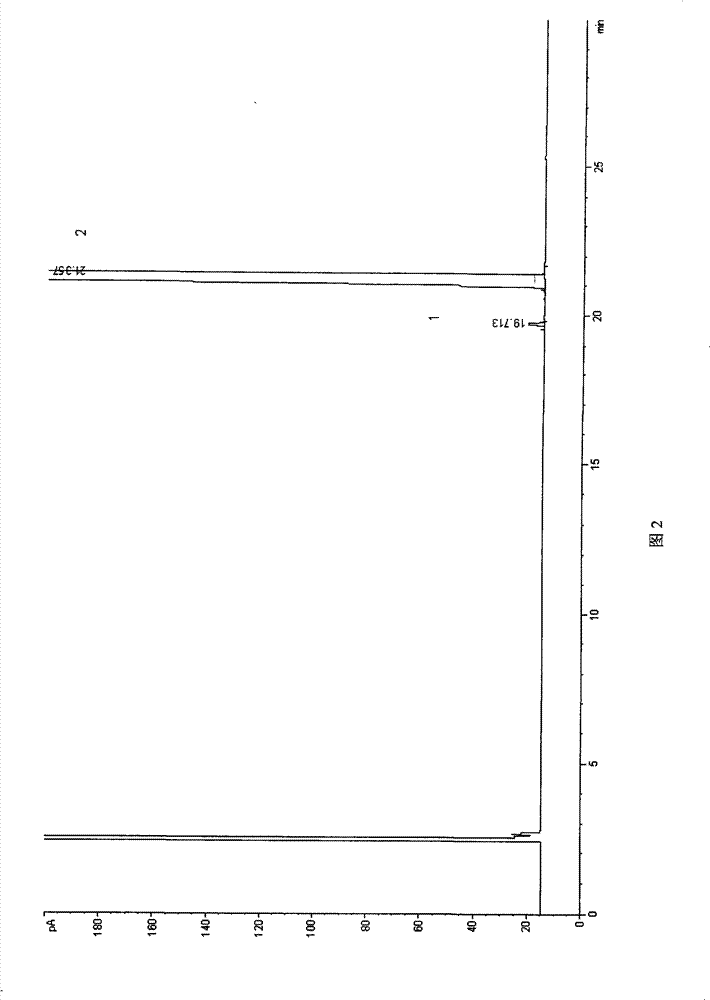
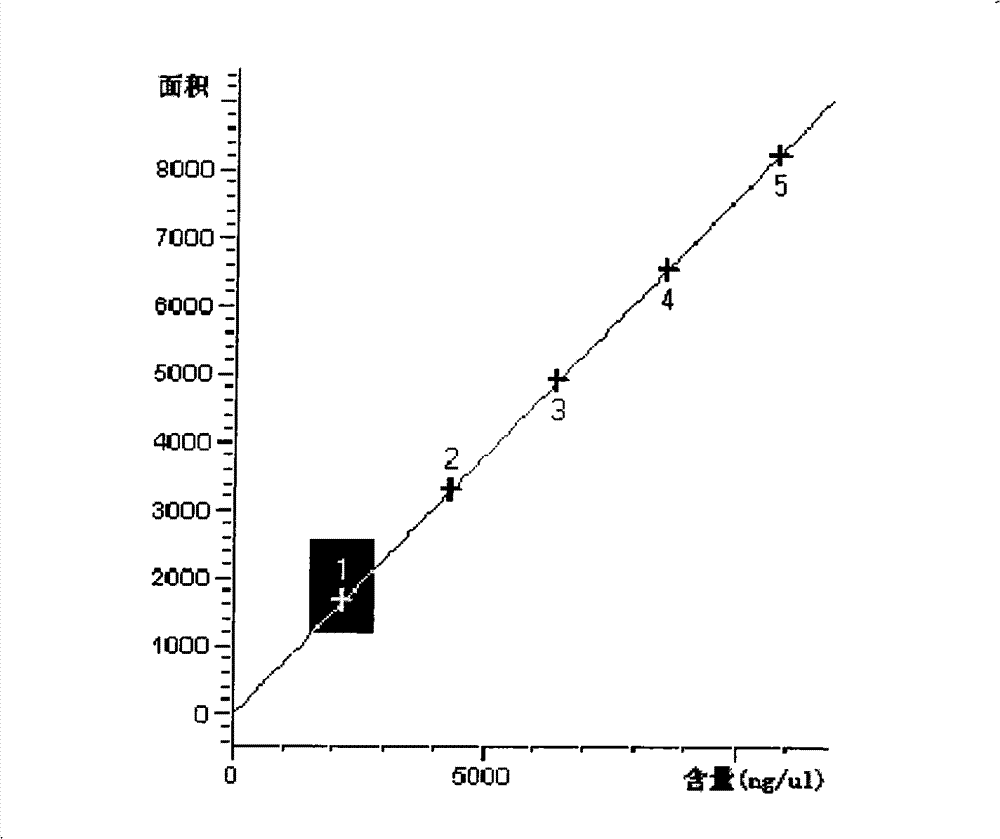
Method for constructing Compound Xueshuantong preparation HPLC fingerprint pattern and method standard fingerprint pattern thereof
ActiveCN1670529AMonitor qualityMonitor stabilityTesting dairy productsBiological testingSalvia miltiorrhizaHplc fingerprint
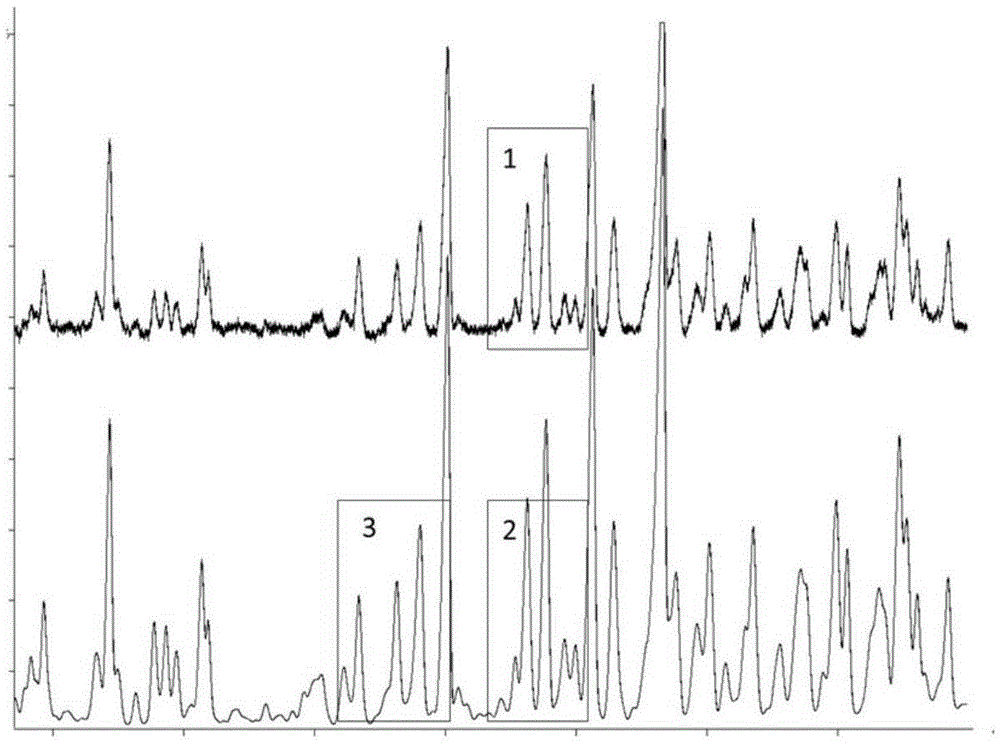
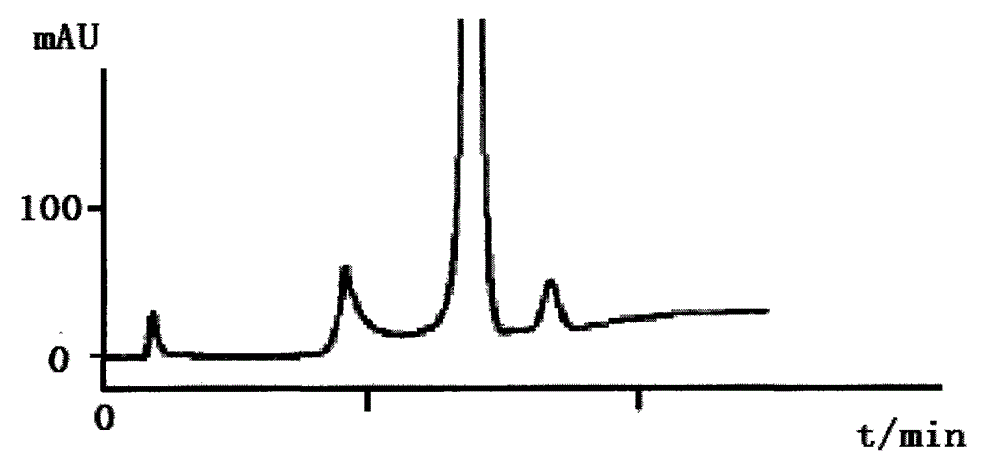
This invention relates to compound thrombus-relaxing agent fingerprint spectrum recreation method by use of natural plants medicinal materials, which comprises the following parts: test solution process; comparing liquid process; measuring with effective liquid chromatograph measurement with chromatograph relative keeping time and peak area as one; computing the test items relative keeping time and relative peak area to get the HPLC fingerprint chromatograph. The required HPLC standard fingerprint chromatograph to notoginseng chromatograph has five common peaks; the one to astragalus root has two common peaks; chromatograph to radix salvia miltiorrhiza has two common peaks; one to scrophularia root has two common peaks; the one to radix salvia miltiorrhiza under 270 nm has three common peaks; the one to scrophularia root has common peak.
Owner:SUN YAT SEN UNIV +1
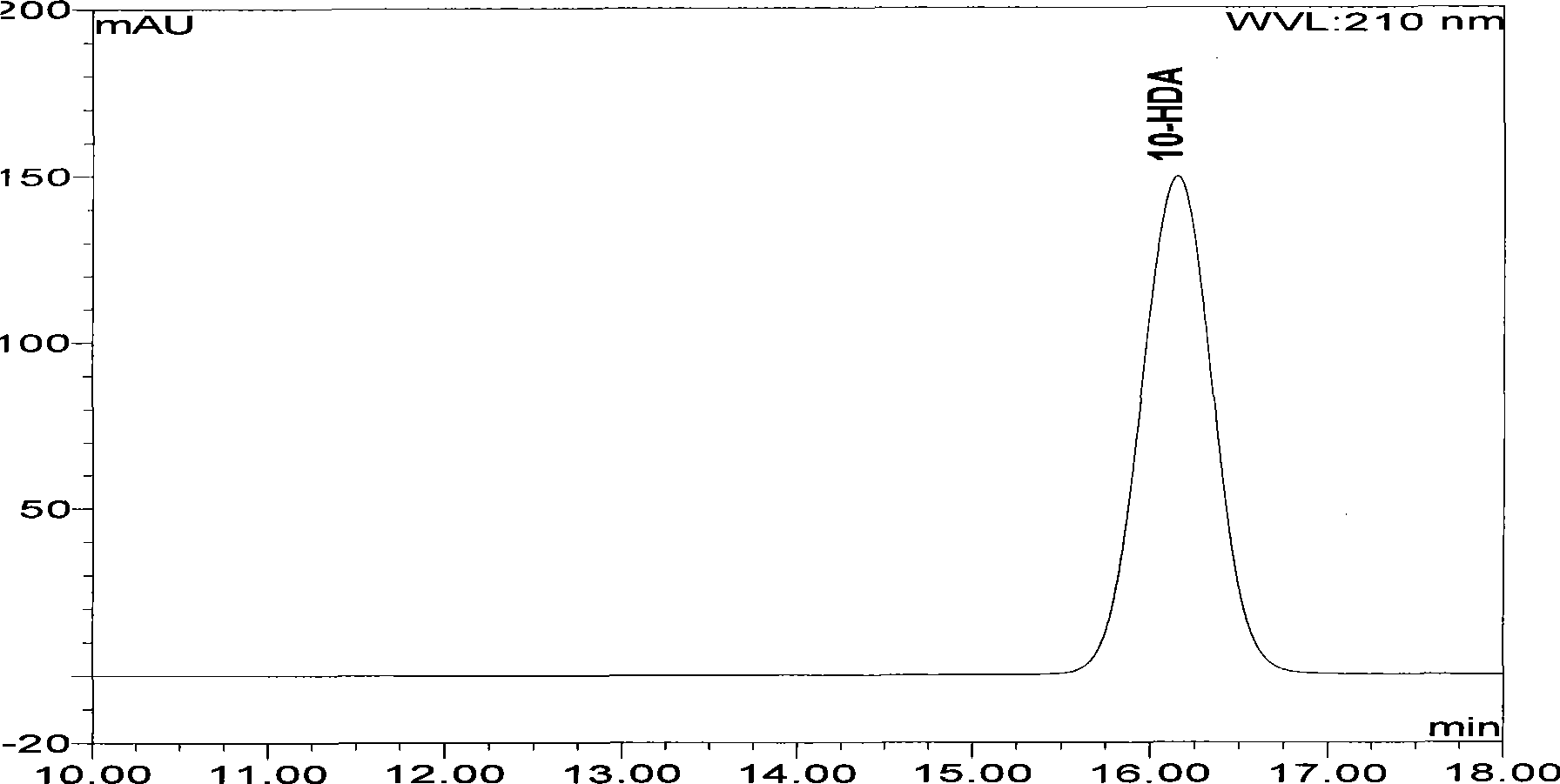
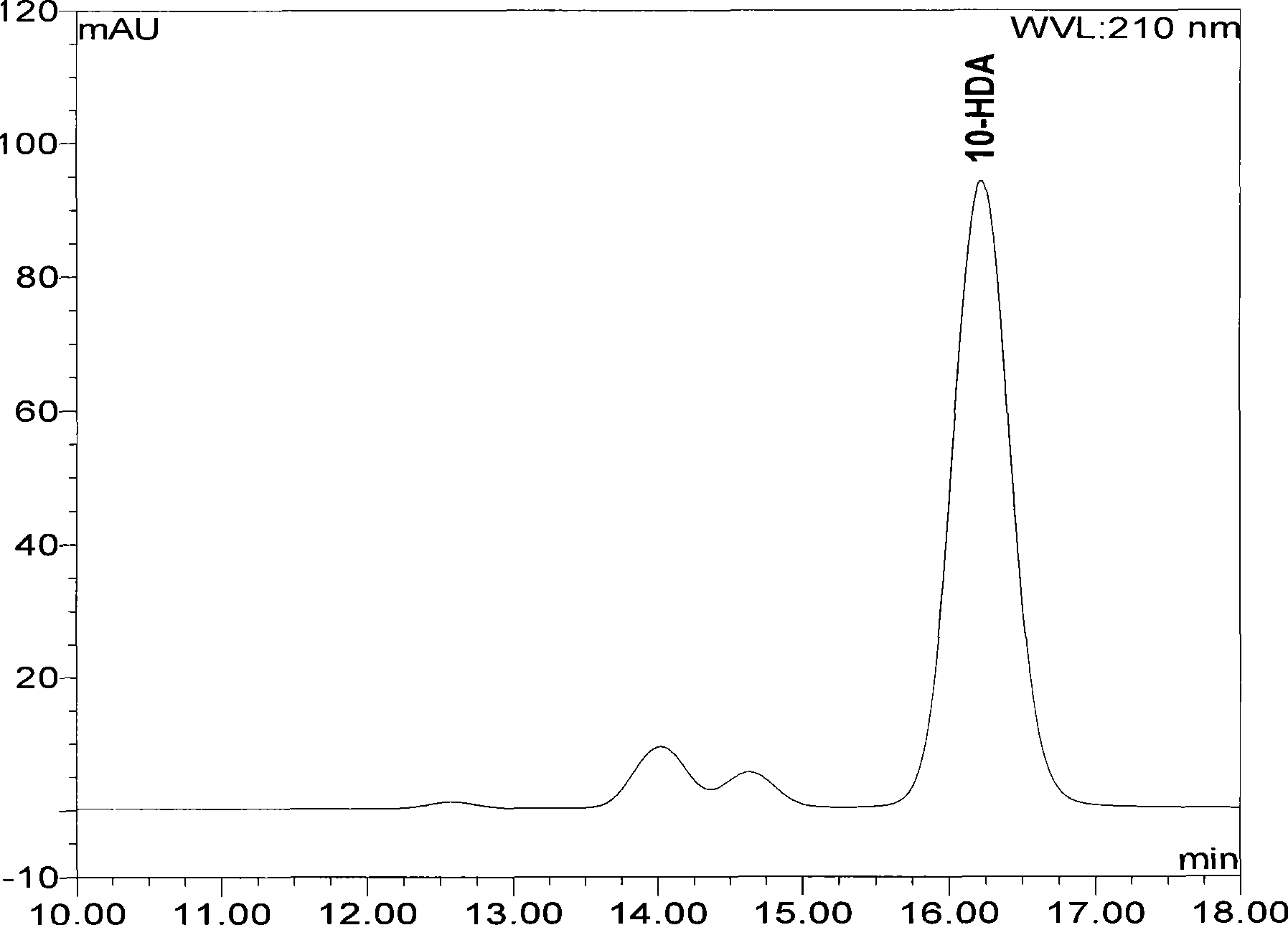
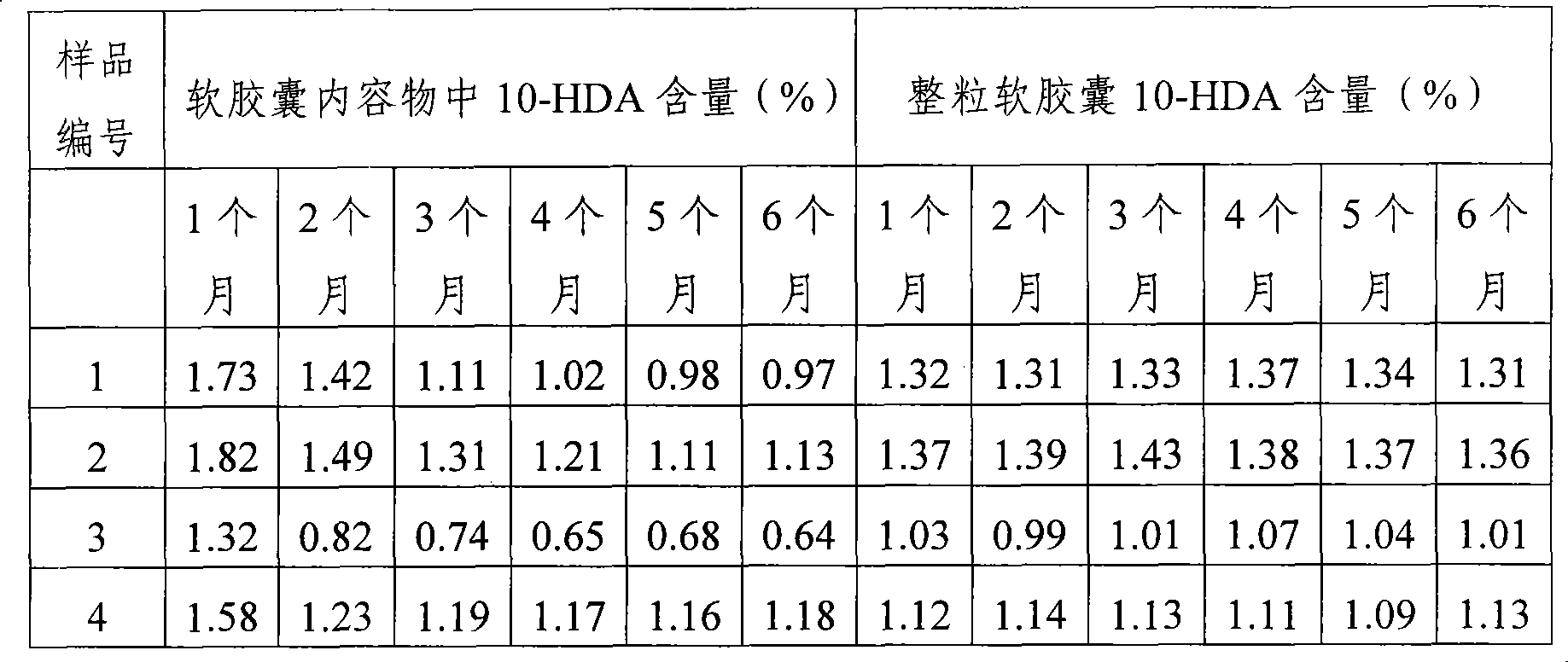
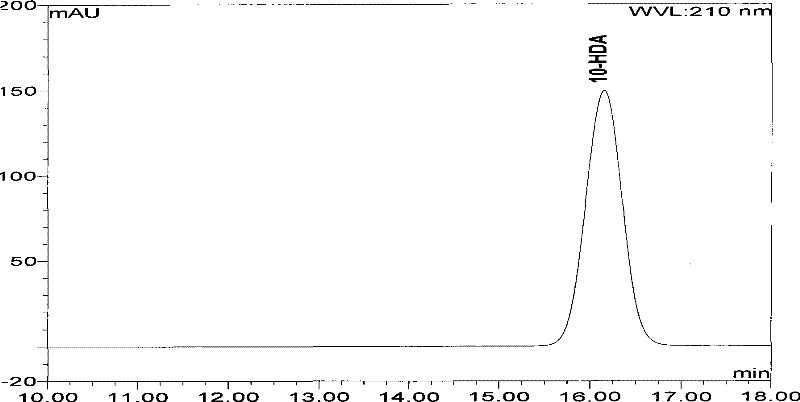
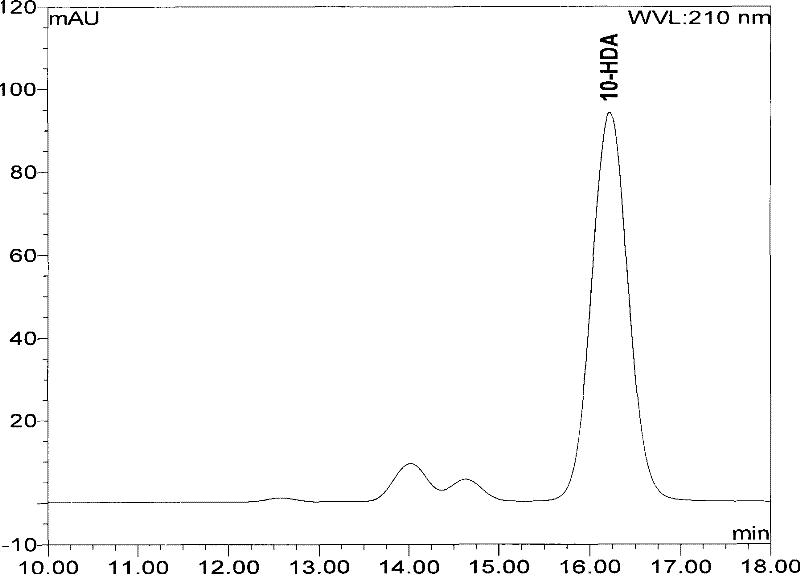
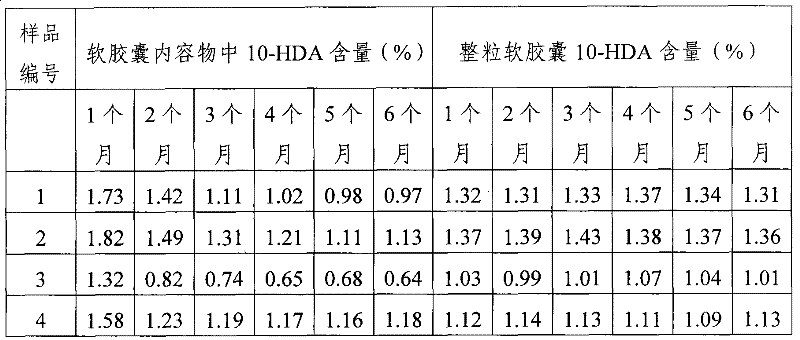
High performance liquid chromatography measuring method for 10-HDA content in royal jelly soft capsule
InactiveCN101504397AThe method has high accuracy and sensitivityGood reproducibilityComponent separationChemistryFormic acid
The invention provides a high performance liquid chromatography method for detecting content of 10-HDA in a royal jelly soft capsule. An alkaline hydrolysis method is adopted for breaking the shell of the soft capsule; and after the pH value is regulated to be less than or equal to 5.0 by an acidity regulator (phosphoric acid, hydrochloric acid, sulfuric acid, formic acid or acetic acid), the anhydrous alcohol is used for ultrasonic extraction. The method has the advantages of effectively detecting the content of the 10-HDA in the royal jelly soft capsule, along with high accuracy and sensitivity and good repeatability.
Owner:BEE RES INST CHINESE ACAD OF AGRI SCI
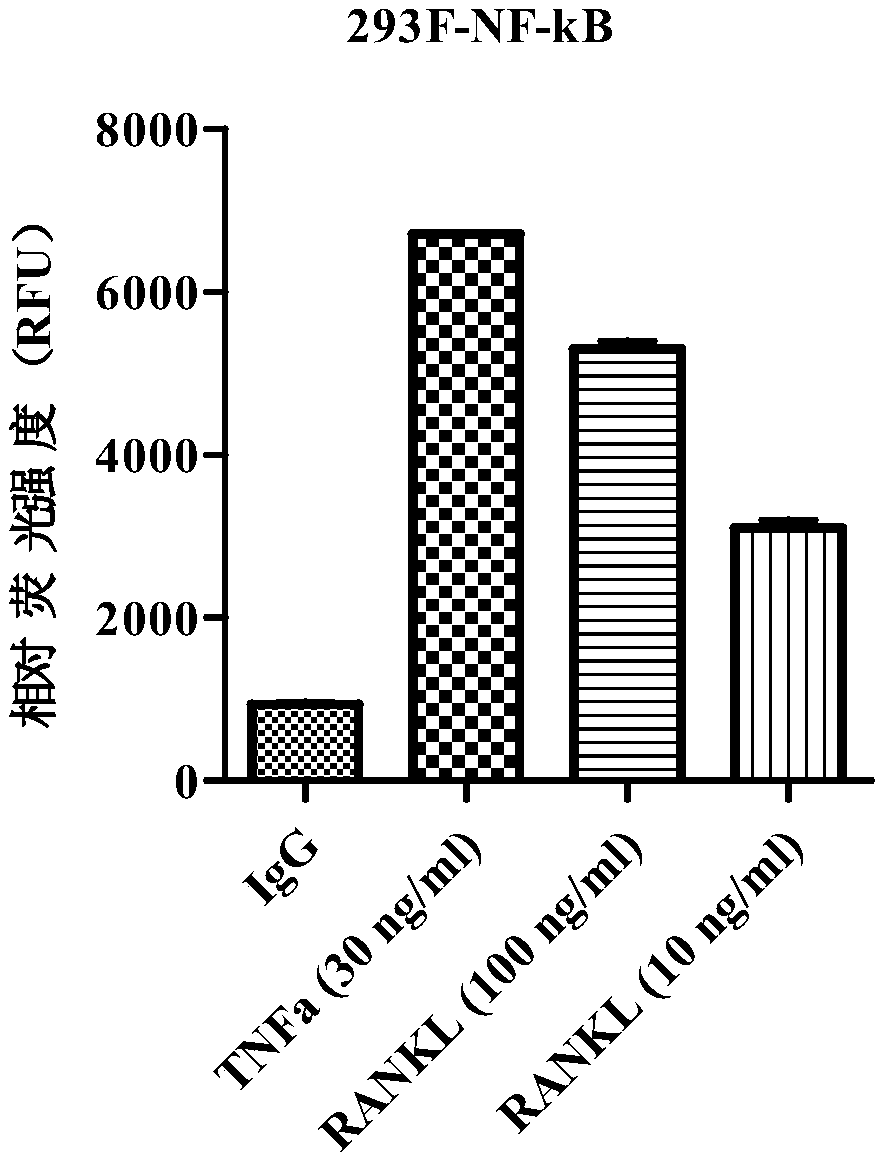
Method for detecting biological activity of RANKL (receptor activator for nuclear factor-KB ligands) targeted therapy medicines
InactiveCN108753920AQuick checkApplicable test qualityMicrobiological testing/measurementTranscription Factor NF-kBFactor ii
The invention provides a method for detecting the biological activity of RANKL (receptor activator for nuclear factor-KB ligands) targeted therapy medicines. The method includes steps of firstly, constructing transcription factor NF-kB (nuclear factor-kB) binding sequences, cloning the NF-kB binding sequences into luciferase reporter gene carriers, and guiding luciferase reporter gene transcription by the aid of the NF-kB binding sequences used as components of starters so as to obtain plasmids with the NF-kB binding sequences and luciferase reporter genes; secondly, transfecting cells by theplasmids with the NF-kB binding sequences and the luciferase reporter genes; thirdly, vaccinating the cells, adding RANKL and the RANKL targeted therapy medicines into the cells and then culturing thecells; fourthly, lysing the cells, adding luciferase substrates into the cells and detecting the fluorescence intensity so as to determine the biological activity of the RANKL targeted therapy medicines. The method has the advantage that the biological activity of the denosumab RANKL targeted therapy medicines can be accurately, easily, feasibly and quickly detected by the aid of the method.
Owner:GUANGDONG ANNPO BIOTECHNOLOGY INC
HPLC-ELSD content determination method for paeoniflorin and albiflori in red paeony roots
InactiveCN105116086AApplicable quality controlStrong specificityComponent separationIsocratic elutionQuality control
Owner:LIAONING UNIVERSITY
Detection method of blood nourishing and brain refreshing preparation
ActiveCN107917966AIdentify quality attributesAchieving Quality EvaluationComponent separationTest articleAdditive ingredient
The invention relates to a detection method of a blood nourishing and brain refreshing preparation. The method is a high performance liquid chromatography and comprises following steps: step 1, preparation of a test solution; step 2, preparation of a control solution; step 3, chromatographic conditions; step 4, content determination: the test solution and the control solution are injected into a high performance liquid chromatograph, and a chromatogram is obtained. According to the provided method, a test article is treated by a solid-phase chromatographic column, ingredients are enriched, purified and then analyzed, and the separation degree of the chromatographic peak and the reproducibility of the method are better guaranteed while the analysis time of the corresponding chromatographicmethod is shorter. The relative quantification mode of a fingerprint spectrum is clearly defined, the control requirements for two absolute quantification indexes for content determination and 19 relative quantification indexes of the fingerprint spectrum are defined, the control indexes are more comprehensive, and more quantized description of quality attributes of products is performed.
Owner:TIANJIN TASLY PHARMA CO LTD
Method for analyzing products for removing alkene
InactiveCN106596745AApplicable quality controlTimely judgment of removal degreeComponent separationGas phaseQuality control
The invention relates to a method for analyzing products for removing alkene, and mainly solves the problems that in the prior art alkene impurity removing degree in existed products for removing alkene can only be determined separately by alkene structure identification experiments and content determination experiments in order to know whether alkene is completely removed, the analysis process takes a long time, and when alkene identification appears error, alkene impurity removing degree in the alkene removing products cannot be determined timely. Gas chromatography parallel double-detector apparatus is used, and the alkene removing products are treated by gas chromatography sample introduction, chromatographic column separation, capillary column outlet current divider, and detection by two parallel different detectors; alkene structure and content information in the products are obtained at the same time, and alkene impurity removing degree in the alkene removing products can be determined timely. The technical scheme can better solve the problems and is suitable for quality control of alkene removing products.
Owner:CHINA PETROLEUM & CHEM CORP +1
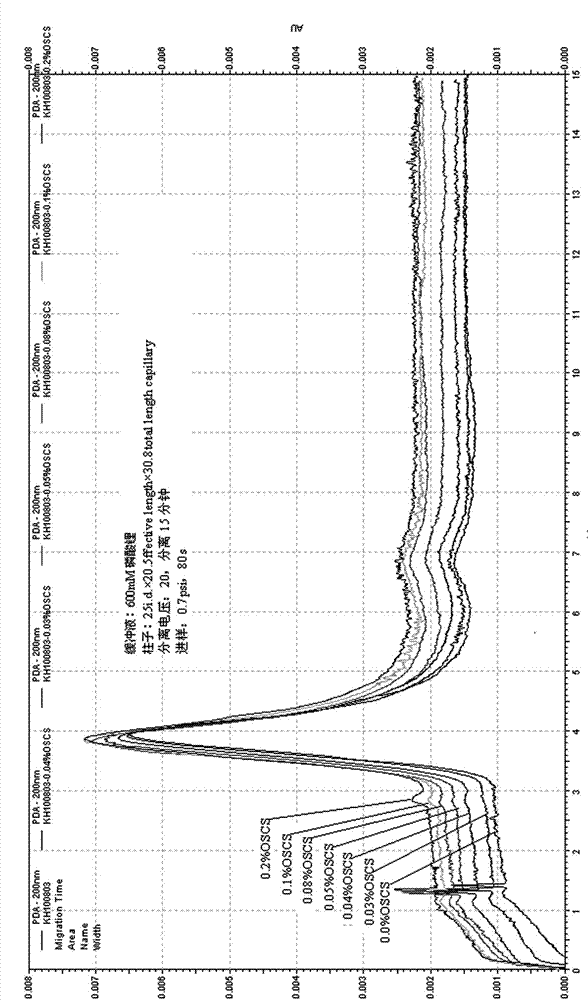
High sensitivity detection method of oversulfated chondroitin sulfate in liquaemin
InactiveCN102830150AImprove product qualityAvoid pollution incidentsMaterial analysis by electric/magnetic meansPhosphateLITHIUM PHOSPHATE
The invention relates to a high sensitivity detection method of oversulfated chondroitin sulfate in liquaemin. The high sensitivity detection method comprises the following steps of 1) preparing a sample liquid and weighting a liquaemin sample; 2) preparing a standard substance solution and using purification water to dissolve a system suitability standard substance, a liquaemin identification standard substance and an oversulfated chondroitin sulfate standard substance into a solution; 3) preparing a buffered solution and preparing a phosphate buffer or a lithium phosphate buffer in a volumetric flask; and 4) selecting a fused quartz capillary, conducting sample introduction analysis on the liquaemin system suitability standard substance and the liquaemin identification standard substance in the phosphate buffer, and enabling a liquaemin peak of the liquaemin system suitability standard substance and a peak of the oversulfated chondroitin sulfate to be completely separated. The high sensitivity detection method has the advantages of being high in detection sensitivity, short in detection time, simple and convenient to operate, good in reproducibility, capability of effectively controlling the oversulfated chondroitin sulfate in the liquaemin, being favorable for further promoting the quality of liquaemin products and avoiding occurrence of liquaemin contamination accidents again.
Owner:DONGYING TIANDONG PHARM CO LTD
High efficiency liquid phase chromatographic analysis method for 2-benzotrinitrozole-4,6-diiso propyl phenol content in nylon 66
InactiveCN1987447AApplicable quality controlGood analytical reproducibilityComponent separationBenzeneAlcohol
The invention uses lytic agent in phenols to dissolve product of nylon 66. After separating agent in alcohols separates nylon 66 out from dissolution liquid, alcoholic solution of containing 2-benzotriazol-4, 6- phenol diisopropyl benzene is obtained. Using high performance liquid chromatography analyzes content of 2-benzotriazol-4, 6- phenol diisopropyl benzene in product of nylon 66. The invention can pick up agent of anti ultraviolet of 2-benzotriazol-4, 6- phenol diisopropyl benzene from product of nylon 66 conveniently. Features are: good reproducibility of high performance liquid chromatography analysis, and suitable to control quality of product of nylon 66.
Owner:SHANGHAI CHEM REAGENT RES INST
Enrichment and characterization method of American ginseng polypeptide
ActiveCN111257438AAchieving Deep RepresentationSeparation helpsComponent separationAMERICAN GINSENG ROOTMass Spectrometry-Mass Spectrometry
The invention discloses an enrichment and characterization method of American ginseng polypeptide. The method comprises steps of firstly, preparing American ginseng extracting solution; adding ethyl acetate into the American ginseng extract liquor to obtain extract liquor containing the ethyl acetate and the extract liquor without the ethyl acetate; adding n-butyl alcohol into the extract liquor without the ethyl acetate to obtain the extract liquor containing the n-butyl alcohol and water-bearing layer extract liquor without the n-butyl alcohol; and carrying out ultrafiltration of the water-containing layer extraction liquid, centrifuging, collecting the supernatant, and carrying out HPLC gradient elution to obtain the eluent which is the enriched American ginseng polypeptide. The methodis advantaged in that the enriched polypeptide is characterized by using the mass spectrometry technology and a proteomics analysis database.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Impurity detection method of cefozopran hydrochloride
PendingCN113552250AApplicable quality controlEasy to separateComponent separationAgainst vector-borne diseasesCefozopran HydrochlorideAqueous solution
The invention discloses an impurity detection method of cefozopran hydrochloride. Chromatographic conditions are as follows: an ACE Excel CN-ES chromatographic column and a Waters Atlants T3C18 chromatographic column are connected in series; methanol and acetonitrile in a volume ratio of (20-30): (70-80) are taken as a mobile phase A, and a triethylamine aqueous solution with a mass concentration of 0.3-0.5% is taken as a mobile phase B; and the volume ratio of the mobile phase A to the mobile phase B is (20-40): (60-80). By adopting the method provided by the invention, the separation degree of each peak of the cefozopran hydrochloride bulk drug is good, the separation degree is greater than 1.5, and the tailing factor is 0.8-1.2. The method is also suitable for detection of various damaged samples with more impurities, and is more beneficial to quality monitoring of cefozopran hydrochloride.
Owner:HAINAN HAILING CHEMIPHARMA CORP
Method for high sensitivity detection of oversulfated chondroitin sulfate and dermatan sulfate in heparin sodium
The invention relates to a method for high sensitivity detection of oversulfated chondroitin sulfate and dermatan sulfate in heparin sodium. A gradient elution method is used for elution, a CIM-QA monolithic column with the diameter of 12mm and height of 3mm is used, ultrapure water is used as a mobile phase A, a high-purity water mixed solution is used as a mobile phase B, and the mixed solution comprises lithium phosphate, dimethyl formamide, glycol and high purity water. The method has the characteristics of low equipment investment, low detection cost and simple processes, has high detection sensitivity, short detection time and good reappearance, can be used for tracking detection of oversulfated chondroitin sulfate and dermatan sulfate and is suitable for quality control in large scale production.
Owner:DONGYING TIANDONG PHARM CO LTD
Analysis method for content of 5-chloro-2-methoxycarbonyl-1-indanone ester
The invention belongs to the technical field of chemical analysis and relates to ananalysis method for the content of 5-chloro-2-methoxycarbonyl-1-indanone ester. According to the analysis method, high performance liquid chromatography is adopted to detect the content of the 5-chloro-2-methoxycarbonyl-1-indanone ester; an SBC8 reversed phase chromatographic column is adopted as a chromatographic column; the temperature of the chromatographic column is 30 DEG C to 50 DEG C; a mobile phase is a mixed system of acetonitrile and ammonium carboxylate; the detection wavelength is 230 nm and 270 nm;impurities, a main peak and a solvent peak can be completely separated; the chromatographic peak shape is good; the retention time is stable; the integral calculation result is accurate; the repeatability is good; the reliability of the obtained result is high; and the method is particularly suitable for quality control of pesticide raw drug intermediate products.
Owner:JINGBO AGROCHEM TECH CO LTD
Argatroban intermediate as well as preparation method and application thereof
InactiveCN111961114AApplicable quality controlHigh purityPeptide preparation methodsBulk chemical productionSulfonyl chlorideTert-Butyloxycarbonyl protecting group
The invention discloses an argatroban intermediate as well as a preparation method and application thereof. The preparation method of the intermediate comprises the following steps: S1, carrying out amidation reaction on N[alpha]-BOC-N[omega]-Cbz-L-arginine shown in a formula 1 and (2R,4R)-4-methyl-2-piperidine carboxylic acid ethyl ester shown in a formula 2 under the action of isobutyl chloroformate to obtain a compound shown in a formula 3; S2, removing tert-butyloxycarbonyl from the compound shown in the formula 3 to obtain a compound shown in a formula 4; and S3, carrying out condensationreaction on the compound shown in the formula 4 and 3-methyl-8-quinoline sulfonyl chloride shown in a formula 5 under an alkaline condition to obtain a compound shown in a formula 6. The benzyloxycarbonyl is adopted to protect guanidyl, structures of all intermediate compounds are not reported in literature, and the key intermediate compound 6 after the protecting group is changed is easy to crystallize to form solid, so that the purity is improved, the quality is easy to control, and the industrial production is facilitated.
Owner:YANGZHOU ZHONGBAO PHARMA
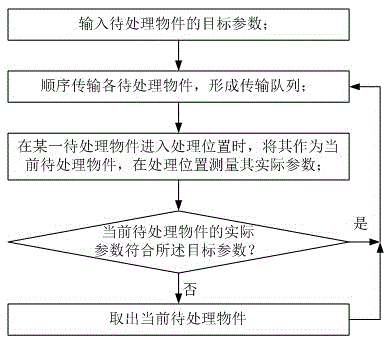
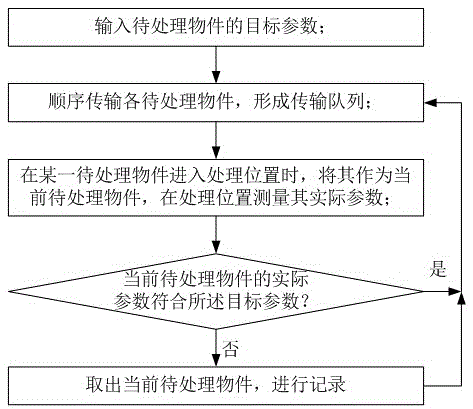
Automatic controlling method
InactiveCN105527950AApplicable quality controlSave human resourcesProgramme total factory controlTablet computerProduction line
The invention provides an automatic controlling method, comprising the following steps: S0, inputting a target parameter of objects to be processed; S1, sequentially transmitting the objects to be processed to form a transmission queue; S2, when an object to be processed enters the processing position, measuring the actual parameter of the object as a current object to be processed at the processing position; S3, judging whether the actual parameter of the current object to be processed complies to the target parameter or not, if so, continuously executing step S1, otherwise, executing step S4; and S4, taking out the current object to be processed, and continuously executing step S1. By adopting the solution, defective objects or rejects can be automatically taken out by comparing and judging with the target parameter, so that a product line can be upgraded by automatic control, and manpower resources are saved; and the method is particularly suitable for quality control of electronic products such as mobile phones, tablet computers and televisions, and is also suitable for quality control of mechanical products.
Owner:SHENZHEN AIYIRUI TECH CO LTD
A high-performance liquid chromatography method for determining the content of 10-hda in royal jelly soft capsules
InactiveCN101504397BSolve the problem of unstable 10-HDA contentApplicable quality controlComponent separationHplc methodPhosphoric acid
The invention provides a high-performance liquid chromatography determination method for determining the content of 10-HDA in the royal jelly soft capsule. The shell of the soft capsule is destroyed by alkaline hydrolysis, adjusted to pH ≤ 5.0 with an acidity regulator (phosphoric acid, hydrochloric acid, sulfuric acid, formic acid, acetic acid), and extracted with absolute ethanol on an ultrasonic wave. The method adopted in the invention can effectively measure the content of 10-HDA in the royal jelly soft capsule, and the method has high accuracy, high sensitivity and good reproducibility.
Owner:BEE RES INST CHINESE ACAD OF AGRI SCI
Method for constructing Compound Xueshuantong preparation HPLC fingerprint pattern
ActiveCN1670529BMonitor qualityMonitor stabilityTesting dairy productsBiological testingSalvia miltiorrhizaHplc fingerprint
Owner:SUN YAT SEN UNIV +1
A kind of detection method of anti-lung cancer sea Mu recipe fingerprint
ActiveCN111398454BComprehensive detection effectComprehensive evaluationComponent separationAgainst vector-borne diseasesPharmacy medicineFingerprint detection
The invention discloses a method for detecting the fingerprint of anti-lung cancer sea mulberry recipe, comprising the following steps: step 1: preparation of a reference peak solution; step 2: preparation of a test solution of anti-lung cancer ha mu recipe; step 3: separately precise Inject the quantitative reference peak solution and the test solution into the liquid chromatograph, and record the chromatogram; Step 4: Export the Haimu Fang fingerprint instrument obtained in step 3, and import it into the Chinese medicine chromatographic fingerprint similarity evaluation system, select different The chromatographic peaks that exist in the chromatograms of the batches of Haimufang tested are regarded as common peaks, and the average value calculation method is used to generate the control fingerprint of Haimufang, and the relative retention time and relative peak area of each common peak are calculated. The fingerprint spectrum of the Haimu prescription provided by the invention can comprehensively and objectively characterize the quality of the Haimu prescription, and is beneficial to comprehensively monitor the quality of the medicine. The fingerprint detection method provided by the invention has the advantages of simple method, stability, high precision, good reproducibility and the like.
Owner:QINGDAO MARINE BIOPHARMACEUTICAL RES INST +1
A kind of quality detection method of Yupingfeng granule
ActiveCN109239220BApplicable quality controlFew stepsComponent separationRepeatabilityComputer science
The invention belongs to the field of medicinal material detection, and in particular relates to a quality detection method for Yupingfeng particles. The quality detection of the Yupingfeng particlescomprises character detection, thin-layer chromatography detection, fingerprint spectrum detection and content limitation detection. The quality detection method for the Yupingfeng particles providedby the invention can authenticate two traditional Chinese medicines: Astragalus membranaceus and Radix saposhnikoviae at a time via a method by using thin-layer chromatography, and is high in accuracyand strong in practicability; the fingerprint spectrum and component content of the Yupingfeng particles can be measured in a unified manner by using ultra-high performance liquid chromatography; viatechnological investigation, the content measurement method provided by the invention is high in accuracy, good in stability and excellent in repeatability, significantly reduces analysis and detection time, and has great significance in setting of Yupingfeng particle quality standard.
Owner:SINOPHARM GUANGDONG GLOBAL PHARMA CO LTD +1
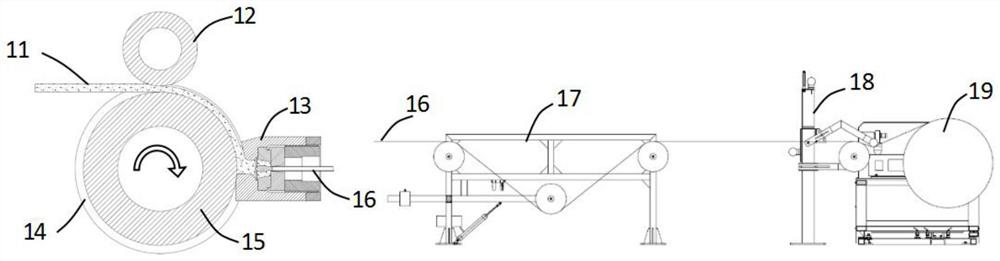
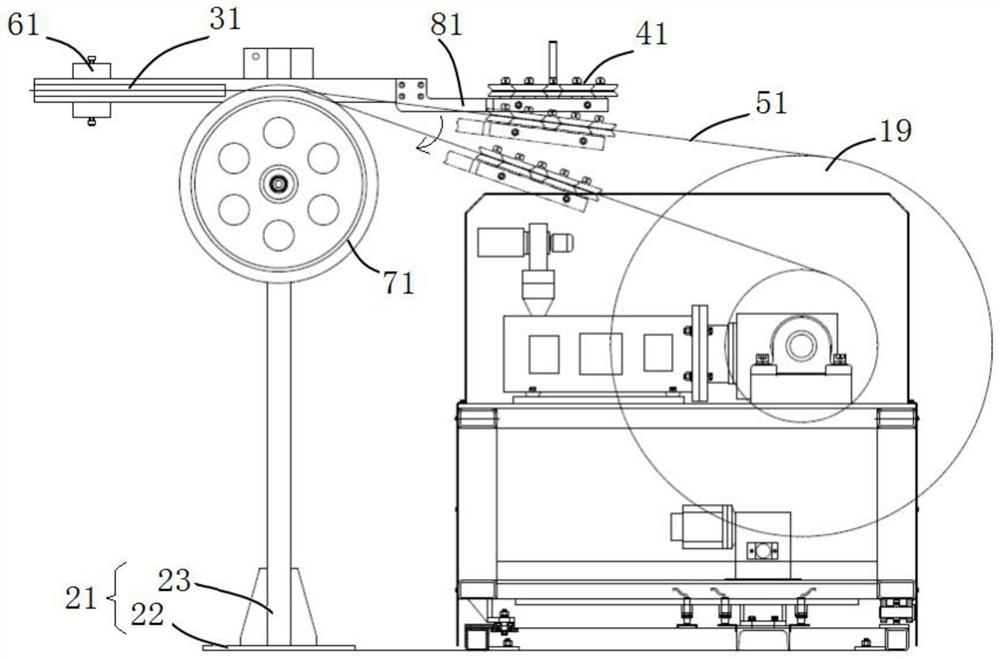
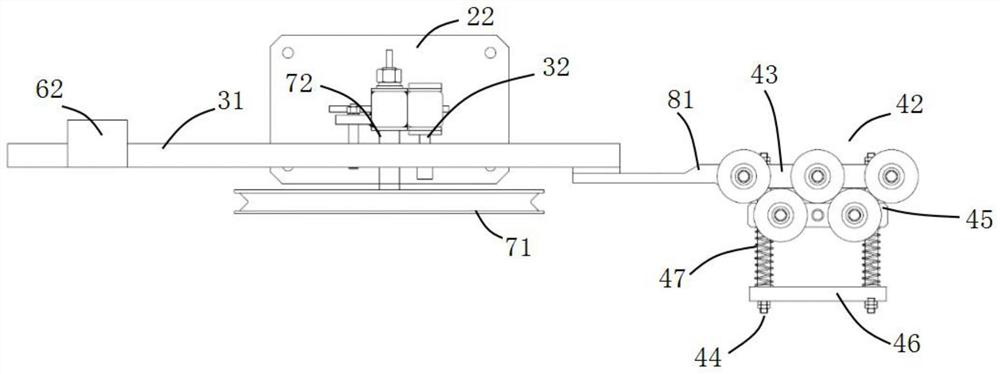
Roundness correction device for circular tube and continuous extrusion winding and unwinding system for circular tube
The invention discloses a round pipe roundness correction device which comprises a support, a swing rod, a round pipe correction assembly and a balance assembly, the round pipe correction assembly is located at the feeding end of a take-up machine and can conduct roundness correction on a round pipe to be wound, and meanwhile the balance assembly can balance the acting force of the wound round pipe on the take-up machine on the swing rod. The circular pipe guided out of the circular pipe correction assembly is always tangent to a reel of the take-up machine, and the circular pipe subjected to roundness correction is effectively prevented from bending and deforming again. The device has roundness correction and anti-bending functions, solves the problems that the roundness of the round aluminum pipe becomes poor and the dimensional precision is reduced due to multi-pass bending deformation in the vertical direction after the round aluminum pipe is extruded and before the round aluminum pipe is wound, is beneficial to realizing continuous extrusion winding displacement of the high-precision round pipe, and is suitable for quality control of the refrigeration round aluminum pipe. The circular tube continuous extrusion take-up and winding displacement system comprises circular tube forming equipment, a take-up machine and the circular tube roundness correction device.
Owner:大连康丰科技有限公司
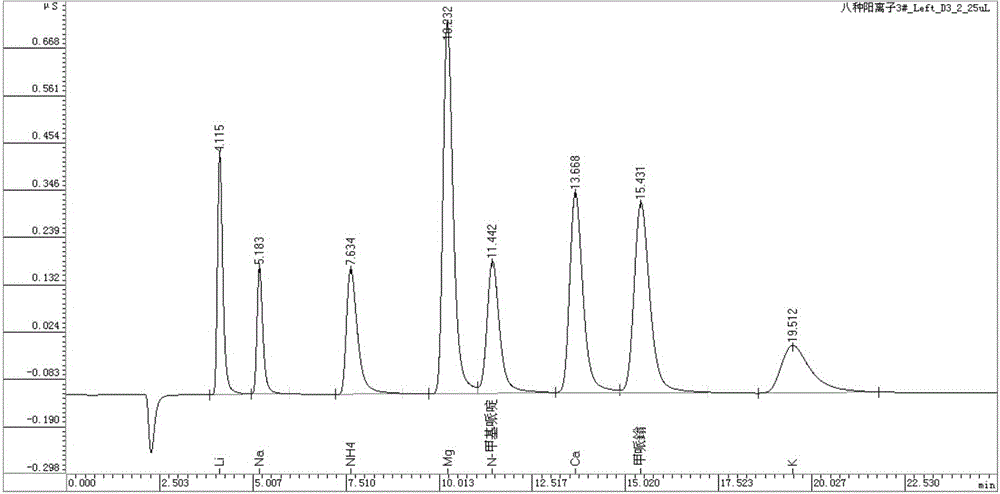
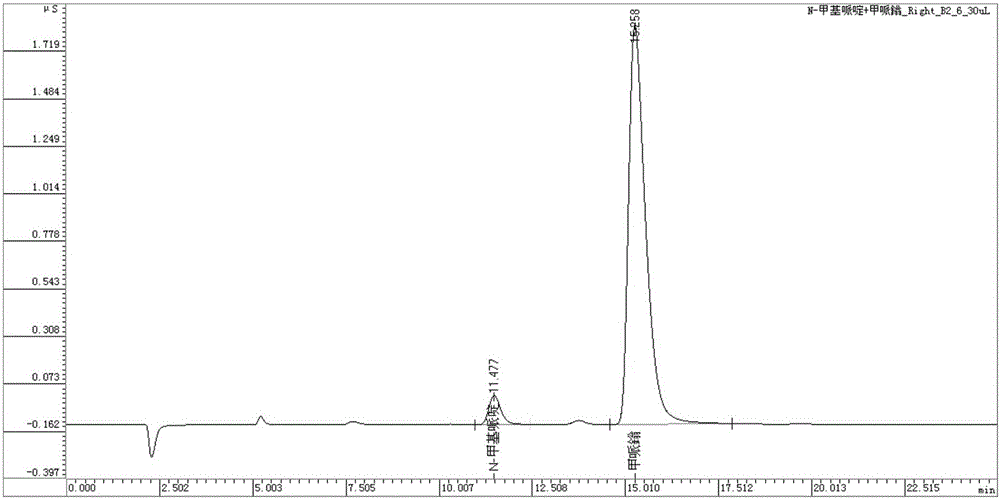
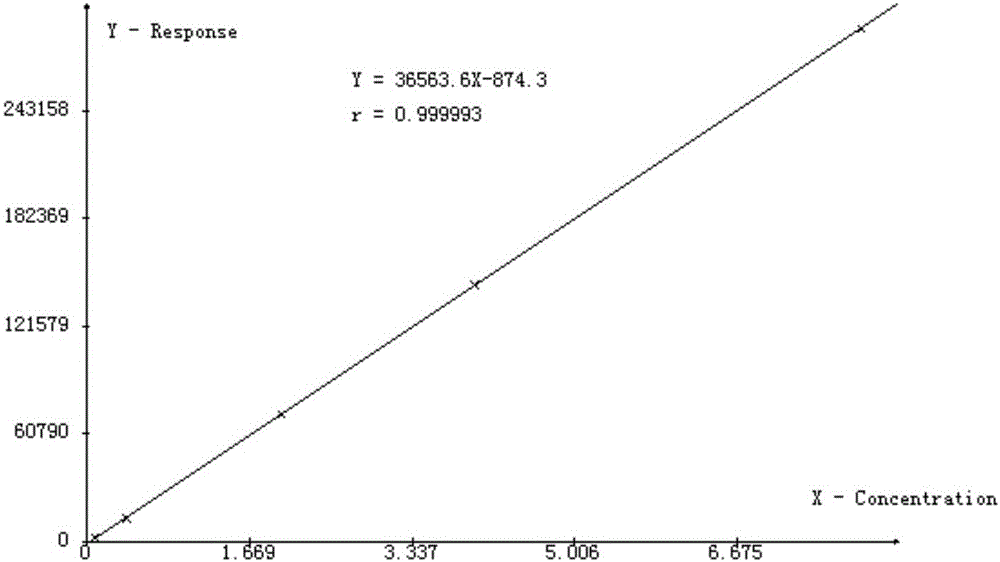
An ion chromatography method for the simultaneous determination of main component mepiperium and its impurity n-methylpiperidine in pesticides
ActiveCN103808845BApplicable quality controlMeet process control needsComponent separationIon chromatographyN-methylpiperidine
The invention discloses an ion chromatography method for simultaneously detecting mepiquat as a main component and N-methylpiperidine as an impurity in a pesticide. The ion chromatography method comprises the following steps of (1) setting the temperature of a column oven to be 45 DEG C; (2) setting the sample injection amount to be 25 microliters; (3) setting applied suppression current to be 15mA; (4) preparing leacheate; (5) preparing a standard solution; (6) analyzing a mixed standard solution with the N-methylpiperidine and the mepiquat; (7) diluting a liquid sample by 10,000 times as a sample solution for later use; (8) filtering a post-treated sample through a disposable needle type filter, putting the sample into an ion chromatography for analysis to measure the contents of negative N-methylpiperidine and mepiquat ions. The method disclosed by the invention is quick, simple and accurate, is suitable for quality control in pesticide production and can meet a requirement on technical control in a pesticide production process.
Owner:ANHUI WAYEE SCI & TECH CO LTD
A kind of detection method of blood-nourishing brain preparation
ActiveCN107917966BIdentify quality attributesAchieving Quality EvaluationComponent separationTest sampleBiochemistry
The present invention relates to a detection method of nourishing serum and brain preparation, said method is high-performance liquid chromatography, comprising the following steps: step 1, preparation of test solution, step 2, preparation of reference solution, step 3, chromatography Condition; Step 4, assay, need testing solution and reference substance solution are injected high-performance liquid chromatograph, obtain chromatogram. In the method provided by the present invention, after the test sample is processed by a solid-phase chromatographic column, the components are analyzed after enrichment and purification, so that the analysis time of the corresponding chromatographic method is shorter, and at the same time, the separation of chromatographic peaks and the weight of the method are guaranteed. and the present invention clearly defines the relative quantitative mode of the fingerprint, and clearly defines the control requirements of the absolute quantitative index (2) of the content determination and the relative quantitative index (19) of the fingerprint, and the control index is more comprehensive, to The quality attributes of the product are described more quantitatively.
Owner:TIANJIN TASLY PHARMA CO LTD
Industrial automatic intelligent control method
InactiveCN105487511ASave human resourcesApplicable quality controlProgramme total factory controlAutomatic controlQuality control
The invention provides an industrial automatic intelligent control method. The method comprises the following steps: S0, inputting object parameters of objects to be processed; S1, sequentially transmitting each object to be processed, and forming a transmission queue; S2, when a certain object to be processed enters a processing position, taking the object as a current object to be processed, and measuring actual parameters of the current object to be processed at the processing position; S3, determining whether the actual parameters of the current object to be processed accord with the object parameters, if so, continuously executing step S1, and otherwise, executing step S4; and S4, removing the current object to be processed, performing recording, and continuously executing step S1. By using the scheme provided by the invention, through comparison and determination of the object parameters, flawed pieces or defective products can be automatically removed, a product line is upgraded by use of automatic control, and human resources are reduced. The industrial automatic intelligent control method is particularly applied to quality control of such electronic products as mobile phones, tablet computers, televisions and the like and is also suitable for quality control of mechanical products in the same way.
Owner:SHENZHEN AIYIRUI TECH CO LTD
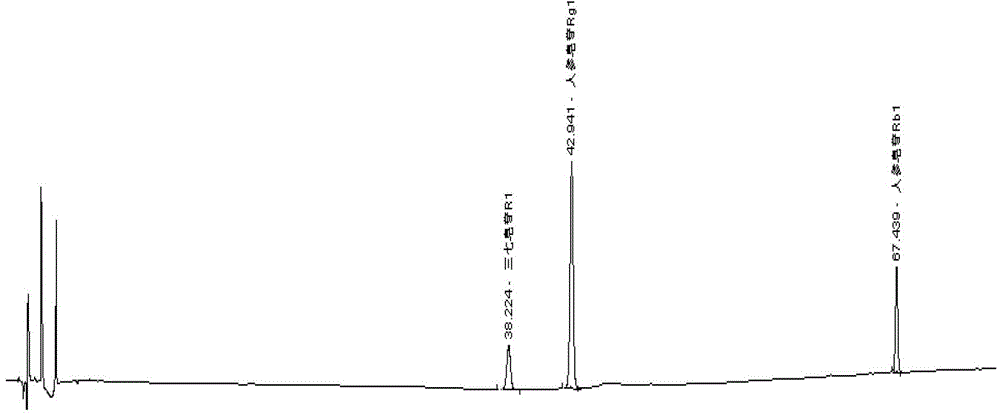
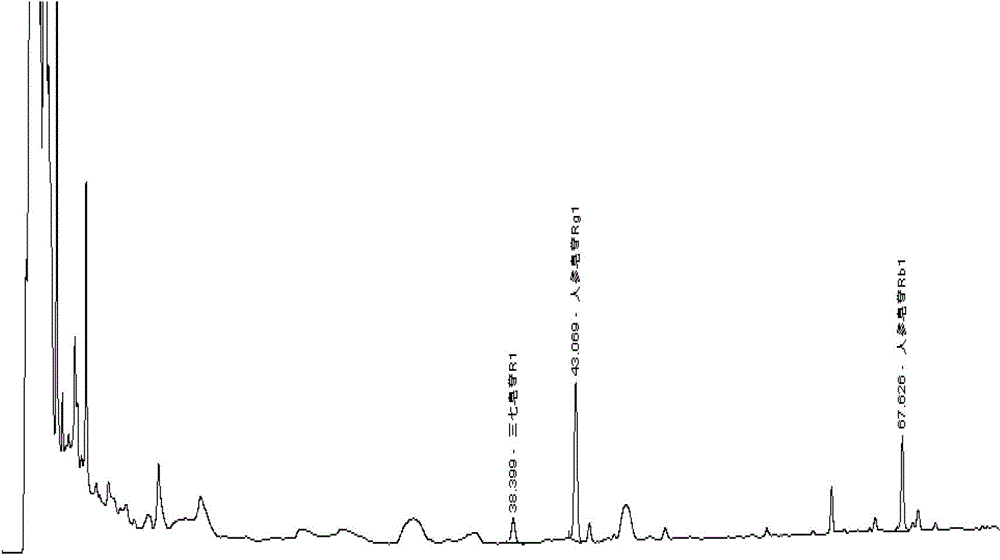
Method for determining content of panax pseudoginseng in musk ointment for promoting blood circulation by removing blood stasis
InactiveCN103499649AApplicable quality controlImprove accuracyComponent separationMedicineRemove blood
The invention relates to a method for determining the content of panax pseudoginseng in musk ointment for promoting blood circulation by removing blood stasis. The method comprises the steps of dissolving ointment with diethyl ether firstly, then adding water for dissolving, extracting Rg1, Rb1 and R1, standing, separating liquid, washing water liquid with the diethyl ether, filtering with dry filter paper and determining the filtrate by using a high performance liquid chromatography. The determination method has relatively high accuracy and precision degree, easiness and convenience for operation, strong specificity and durability and can be used for quality control of the musk ointment for promoting the blood circulation by removing the blood stasis.
Owner:CHONGQING UNISPLENDOUR CHEM
Method for disinfecting surface of embryo egg for producing vaccine
PendingCN113384722AOvercome limitationsEasy to controlViral antigen ingredientsAntiviralsHydrogen peroxideTGE VACCINE
The invention relates to a method for disinfecting the surface of an embryo egg for producing a vaccine. The method comprises the following steps: (1) treating an aqueous hydrogen peroxide solution into a dry fog state, and treating the embryo egg for 40-60 minutes; and (2) spraying the embryo egg with a 75% ethanol solution, and performing secondary disinfection treatment. The vaccine is an antiviral vaccine, and the virus can be cultured by the embryo egg.
Owner:WUHAN INST OF BIOLOGICAL PROD CO LTD
Method for determining content of panax pseudoginseng in musk ointment for promoting blood circulation by removing blood stasis
InactiveCN103499649BApplicable quality controlImprove accuracyComponent separationMedicineRemove blood
The invention relates to a method for determining the content of panax pseudoginseng in musk ointment for promoting blood circulation by removing blood stasis. The method comprises the steps of dissolving ointment with diethyl ether firstly, then adding water for dissolving, extracting Rg1, Rb1 and R1, standing, separating liquid, washing water liquid with the diethyl ether, filtering with dry filter paper and determining the filtrate by using a high performance liquid chromatography. The determination method has relatively high accuracy and precision degree, easiness and convenience for operation, strong specificity and durability and can be used for quality control of the musk ointment for promoting the blood circulation by removing the blood stasis.
Owner:CHONGQING UNISPLENDOUR CHEM
Method for determining content of conivaptan hydrochloride by virtue of high performance liquid chromatography
InactiveCN106442751AEfficient determinationSolve the problem of unstable contentComponent separationColor/spectral properties measurementsPhosphateAcetonitrile
The invention provides a method for determining the content of conivaptan hydrochloride by virtue of a high performance liquid chromatography. The method comprises the following chromatographic conditions: a mobile phase A is acetonitrile, a mobile phase B is a phosphate buffer solution, the concentration of sodium dihydrogen phosphate in the phosphate buffer solution is 0.02mol / L, the mass concentration of sodium lauryl sulfate in the phosphate buffer solution is 0.5%, and the pH value of the phosphate buffer solution is 2.8-3.2. By virtue of the method, the content of a conivaptan hydrochloride raw material drug can be effectively determined, and the method is high in accuracy and sensitivity and good in repeatability. Compared with a traditional method, the method has the characteristics of high efficiency and accuracy.
Owner:蚌埠丰原涂山制药有限公司
Method for determining content of harpagoside in radix scrophulariae medicinal material
PendingCN114814007ASatisfy the linear requirementThe result standard is objectiveComponent separationMedicinal herbsHarpagoside
The invention discloses a method for determining the content of harpagoside in a radix scrophulariae medicinal material, which belongs to the technical field of content determination methods, and comprises the following steps: preparing a standard solution, detecting by adopting an ultrahigh pressure liquid chromatography-tandem single quadrupole mass spectrometry detector, recording a mass spectrum, determining the peak intensity of main ions and adduct ions, calculating the ratio of the intensity, and determining the content of harpagoside in the radix scrophulariae medicinal material according to the content of harpagoside in the radix scrophulariae medicinal material. Drawing a standard curve of the ratio and the content; under the same condition, the test solution is detected, the content of harpagoside in the medicinal material is obtained according to the standard curve, and the method mainly adopts a quantitative method in which the ratio of the ion strength of the components on a single quadrupole mass spectrometry detector replaces a numerical detection signal of an instrument.
Owner:张勇纯
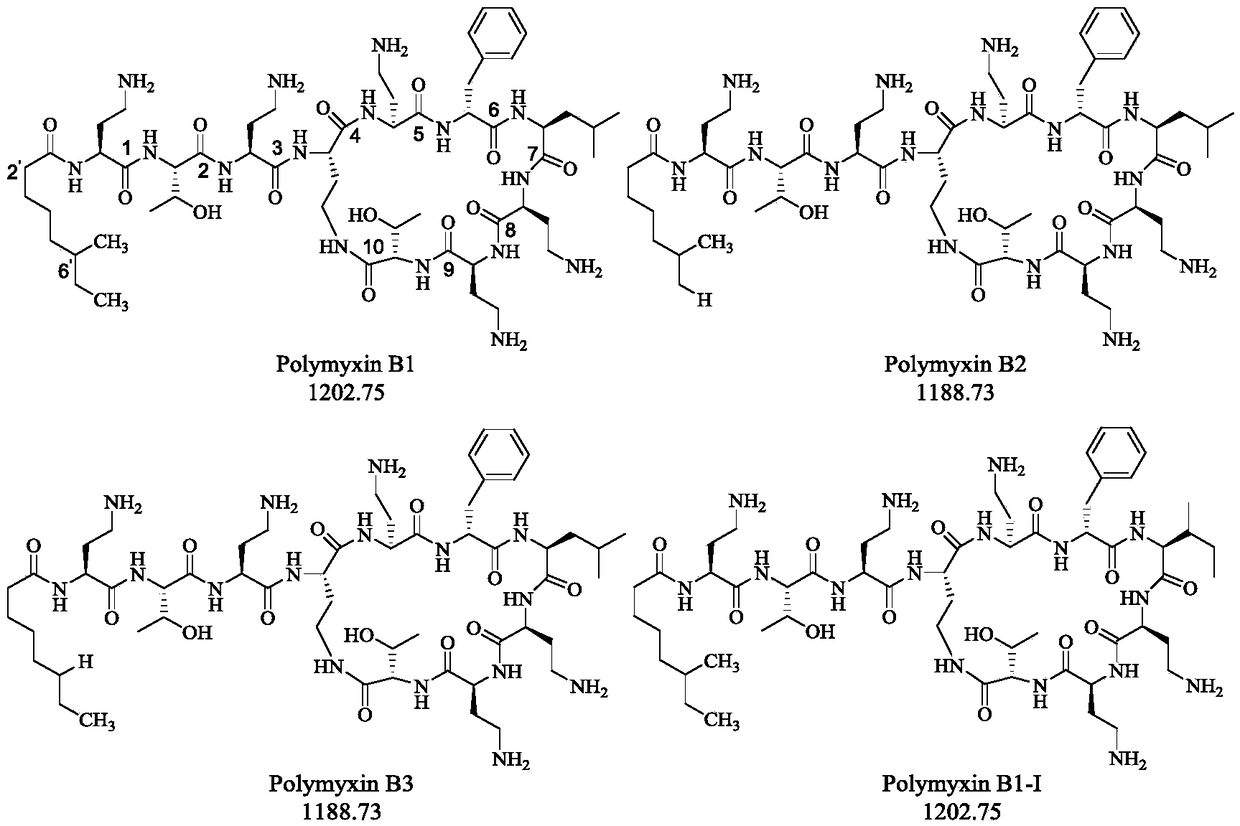
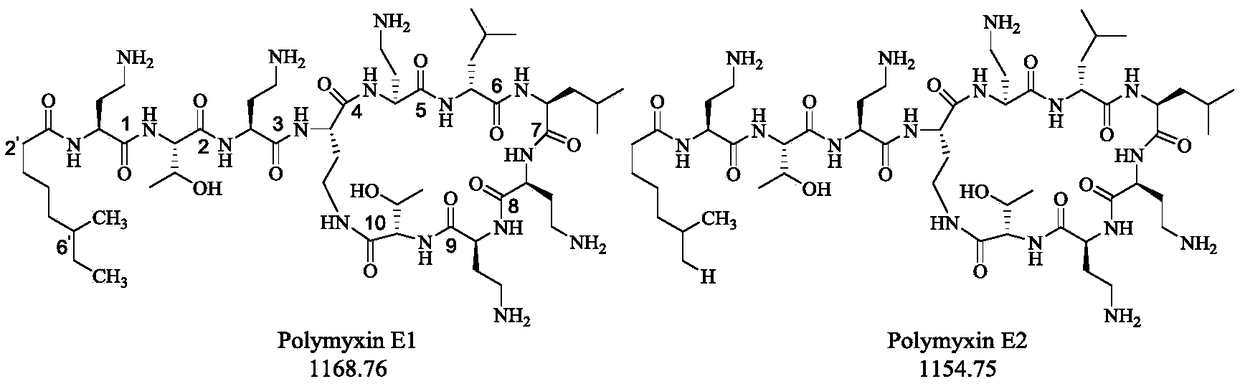
Capillary electrophoretic method for measuring polymyxins antibacterial peptide and running buffer solution thereof
InactiveCN109030610AHigh column efficiencyEasy to operateMaterial analysis by electric/magnetic meansSolventPolymyxin B
The invention relates to a capillary electrophoretic method for measuring polymyxins antibacterial peptide. The method comprises the following steps: dissolving a polymyxins antibacterial peptide preparation in a solvent, directly measuring polymyxins antibacterial peptide through capillary electrophoresis in a running buffer solution, wherein the running buffer solution is a triethanolamine solution or triethylamine solution containing hydroxypropyls cyclodextrin and organic solvent, and the hydroxypropyls cyclodextrin comprises hydroxypropyl-beta-cyclodextrin, hydroxypropyl-alpha-cyclodextrin, hydroxypropyl-gamma-cyclodextrin, and / or sulfated / sulfonated cyclodextrin. The polymyxins antibacterial peptide is analyzed by firstly adopting the triethanolamine solution or triethylamine solution containing hydroxypropyls cyclodextrin and organic solvent in the capillary electrophoresis method, the component and the related substance are good in separation, especially the polymyxin B and E measurement; compared with the liquid phase method, the column efficiency is high, the operation is simple, sample and reagent consumption are less, and the cost is low; the method repeatability is good, and measurement results on samples of different manufacturers are stable and reliable.
Owner:SPH NO 1 BIOCHEM & PHARMA CO LTD
Method for measuring and evaluating emulsifying property of surfactant
ActiveCN109696424AImprove the measurement methodApplicable quality controlScattering properties measurementsTransmissivity measurementsOil phasePeak area
The invention discloses a method for measuring and evaluating the emulsifying property of a surfactant, which comprises the steps of (1) uniformly mixing simulated crude oil and a surfactant solution;(2) measuring a multi-light scattering spectrum; (3) determining an evaluation reference time point T0, the corresponding peak width L0 and the corresponding average light intensity [delta]BS0; (4) calculating the oil phase volume Vo1 and the emulsified oil rate [alpha]o; (5) calculating the peak area width L and the relative average light intensity [delta]BS; (6) determining the derivative relation shown in the description of the dimensionless peak width relative to the dimensionless time, and solving the mean shown in the description of derivatives at different time points; (7) determiningthe derivative of the dimensionless back scattering average light intensity relative to the dimensionless time, and solving the mean shown in the description of derivatives at different time points; and (8) calculating a comprehensive index [eta] to serves as an index for comprehensively evaluating the emulsifying property. The method of the invention comprehensively takes the emulsified oil amount, the emulsion aggregation ability and the coalescence ability into consideration, and has guiding significance for the measurement, evaluation and screening of the surfactant for chemical flooding in the oilfield.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for measuring content of 4,4'-bi(hydroxymethyl) biphenyl (4,4'-BHMBP)
ActiveCN101881757BEasy to separateSimple and fast operationComponent separationCapillary gas chromatographyAutosampler
The invention provides a method for measuring the content of 4,4'-bi(hydroxymethyl) biphenyl (4,4'-BHMBP). In the method, a capillary gas-phase chromatographic column is used for separating the 4,4'-bi(hydroxymethyl) biphenyl, and an automatic sample injector is used for quantifying the 4,4'-bi(hydroxymethyl) biphenyl by adopting an external standard calibration curve method. The method is characterized in that a fixed phase of the capillary gas-phase chromatographic column is 5 percent phenyl 95 percent polydimethylsiloxane, the length of the column is 15-60m, the inner diameter of the column is 0.2-0.5mm, the film thickness of the inner diameter of the column is 0.1-0.6mum, the initial temperature of the column is 60-150 DEG C, and the heating rate is 4-10 DEG C / min; and the sample size of the automatic sample injector is 0.2-5mul, the split ratio is (5:1)-(50:1), and the vaporization temperature is 230-270 DEG C. The method is simple, quick and accurate, is a more ideal qualitative and quantitative analysis method for measuring the content of the 4,4'-BHMBP and is suitable for the quality monitoring of 4,4'-BHMBP products.
Owner:BAOWU CHARCOAL MATERIAL TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com