Impurity detection method of cefozopran hydrochloride
A technology for the detection of cefozopran hydrochloride and its detection method, which is applied in the field of impurity detection of cefozopran hydrochloride, can solve problems such as poor peak shape and detection of impurity content, and achieve good separation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
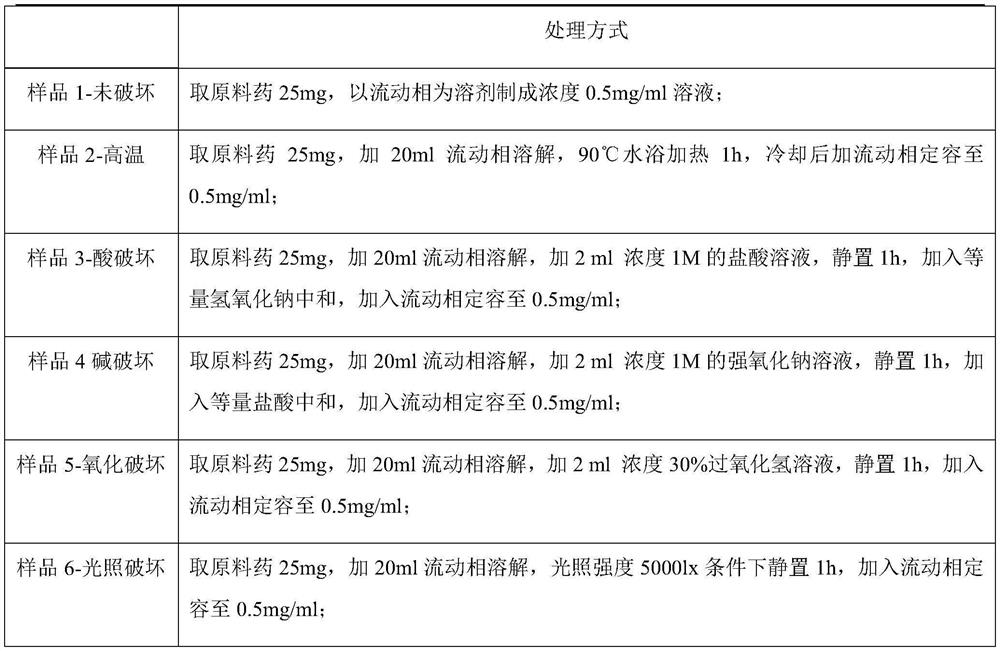
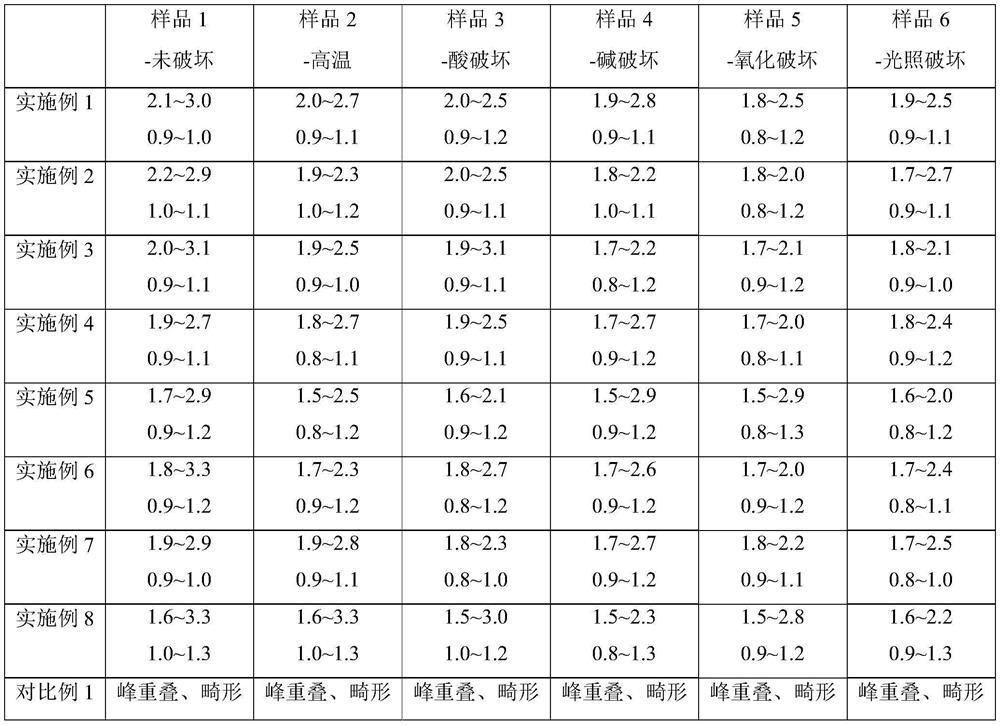
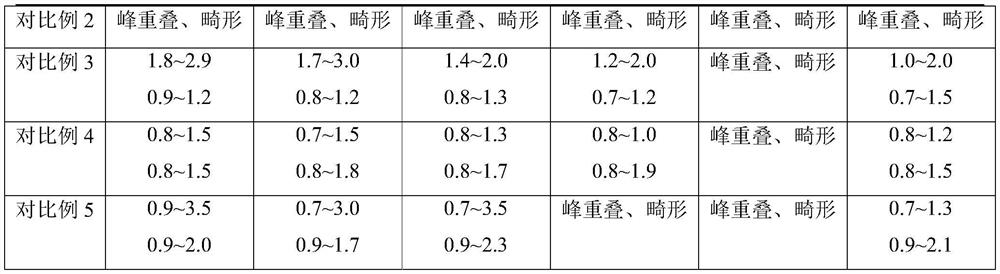
Embodiment 1
[0018] Embodiment 1-cefazolam hydrochloride impurity detection chromatographic conditions:
[0019] Instrument: Shimadzu liquid chromatograph
[0020] Chromatographic column: ACE Excel CN-ES chromatographic column (50mm×3.0mm, 5μm) is connected in series with Waters Atlantis T3 C18 chromatographic column (150mm×4.6mm, 3μm);
[0021] Mobile phase: with methanol and acetonitrile volume ratio 30:70 as mobile phase A, with a mass concentration of 0.5% triethylamine aqueous solution as mobile phase B and mobile phase B with glacial acetic acid to adjust the pH to 4.50; mobile phase A and mobile phase B The volume ratio is 40:60.
[0022] Flow rate 1.5 mL / min.
[0023] The detection wavelength is 254nm.
[0024] The column temperature is 25°C.
Embodiment 2
[0025] Embodiment 2-cefazolam hydrochloride impurity detection chromatographic conditions:
[0026] Instrument: Shimadzu liquid chromatograph
[0027] Chromatographic column: ACE Excel CN-ES chromatographic column (50mm×3.0mm, 5μm) is connected in series with Waters Atlantis T3 C18 chromatographic column (150mm×4.6mm, 3μm);
[0028] Mobile phase: with methanol and acetonitrile volume ratio 20:80 as mobile phase A, with a mass concentration of 0.5% triethylamine aqueous solution as mobile phase B and mobile phase B with glacial acetic acid to adjust the pH to 4.50; mobile phase A and mobile phase B The volume ratio is 20:80.
[0029] Flow rate 1.5 mL / min.
[0030] The detection wavelength is 254nm.
[0031] The column temperature is 25°C.
Embodiment 3
[0032] Embodiment 3-cefazolam hydrochloride impurity detection chromatographic conditions:
[0033] Instrument: Shimadzu liquid chromatograph
[0034] Chromatographic column: ACE Excel CN-ES chromatographic column (50mm×3.0mm, 5μm) is connected in series with Waters Atlantis T3 C18 chromatographic column (150mm×4.6mm, 3μm);
[0035] Mobile phase: with methanol and acetonitrile volume ratio 30:70 as mobile phase A, with a mass concentration of 0.5% triethylamine aqueous solution as mobile phase B and mobile phase B to adjust pH to 3.40 with glacial acetic acid; mobile phase A and mobile phase B The volume ratio is 40:60.
[0036] Flow rate 1.5 mL / min.
[0037] The detection wavelength is 254nm.
[0038] The column temperature is 25°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com