Method for determining content of conivaptan hydrochloride by virtue of high performance liquid chromatography
A technology of conivaptan hydrochloride and high performance liquid chromatography, applied in the field of drug analysis, can solve the problems of increased labor, cumbersome operation, high toxicity, etc., achieves high accuracy and sensitivity, solves content instability and reproducibility Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0066] The selection of experimental example 1 maximum absorption wavelength and chromatographic conditions
[0067] Get conivaptan hydrochloride crude drug, add methyl alcohol and make the solution that contains the conivaptan hydrochloride of 20 μ g about in every 1 milliliter of methanol, measure according to ultraviolet spectrophotometry (two appendix IV A of Chinese Pharmacopoeia 2010 edition), at 200- Wavelength scanning was carried out in the wavelength range of 400nm, and the test results are shown in Table 1 below.
[0068] Table 1 Wavelength scan results
[0069]
[0070]
[0071] The test results show that the methanol solution of this product has maximum absorption at wavelengths of 204nm±2nm, 241nm±2nm, and 276nm±2nm, and the ultraviolet absorption spectrum of the sample is consistent with that of the reference substance. Considering that 204nm is more susceptible to interference and 276nm absorption peak is not obvious, it is preferred to use 240nm as the ...
experiment example 2
[0072] Experimental example 2 specificity
[0073] a, reference substance solution: take about 20 mg of conivaptan hydrochloride reference substance (batch number: 20130223), accurately weighed, put in a 100mL measuring bottle, add solvent (i.e. acetonitrile-phosphate buffer, the volume ratio of acetonitrile to phosphate buffer 57:43) dissolved and diluted to the mark, shake well, that is.
[0074] b. The test solution: take about 20 mg of this product (batch number: 20130129), weigh it accurately, put it in a 100 mL measuring bottle, add solvent to dissolve and dilute to the mark, shake well, and you get it.
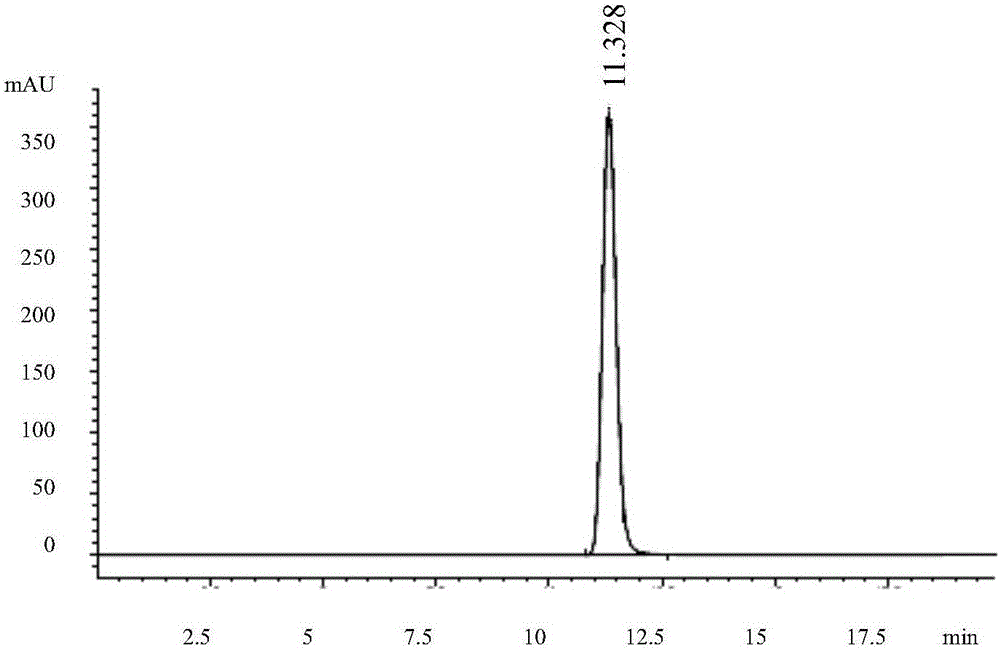
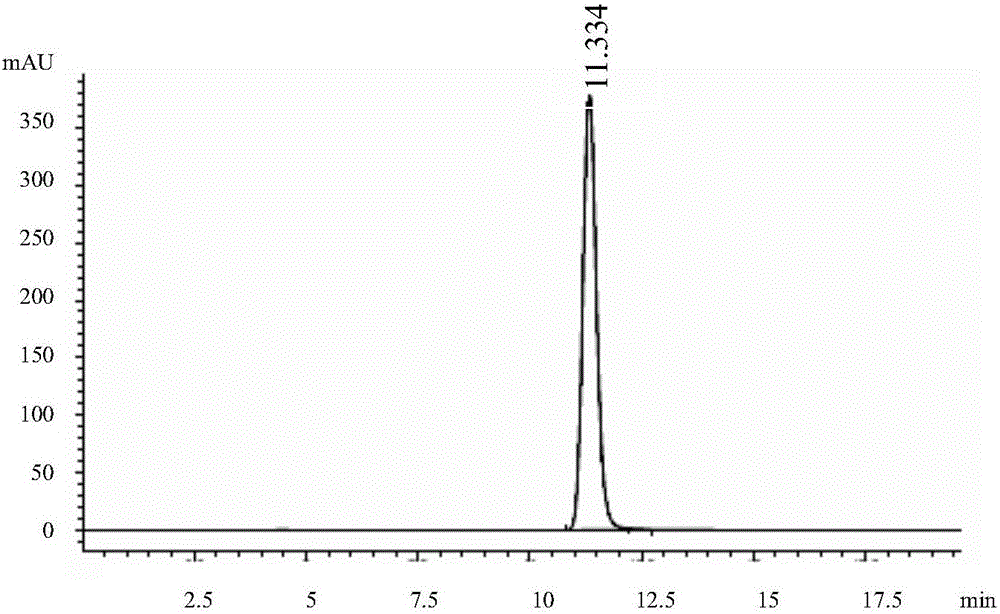
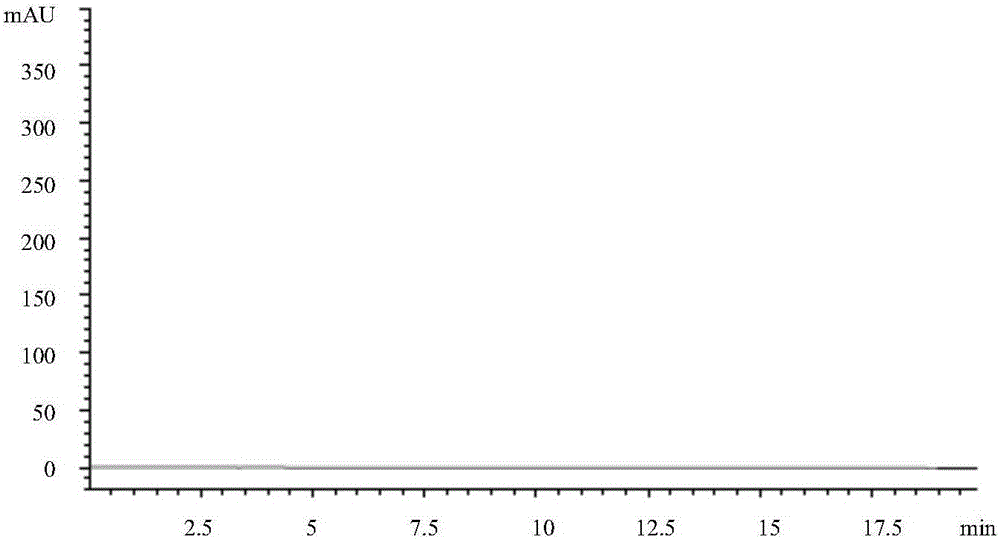
[0075] Get each 10 μ L of solvent, reference substance solution, and need testing solution and inject high performance liquid chromatograph, record chromatogram (see respectively Figure 1-3 ); The result shows that need testing solution and reference substance solution go out peak at identical position, and solvent does not produce any chromatographic peak at this pos...
experiment example 3
[0076] Experimental Example 3 Linearity and Range
[0077] Take 50.12 mg of conivaptan hydrochloride reference substance dried to constant weight, accurately weighed, put in a 25mL measuring bottle, dissolve and dilute to the mark with a solvent, shake well, and use it as the reference substance stock solution; accurately measure the reference substance stock solution Put 0.4mL, 0.8mL, 1.0mL, 1.2mL, 1.4mL and 1.6mL into 10mL measuring bottles respectively, add solvent to dilute to the mark, shake well, and under the above chromatographic conditions, accurately measure 10 μL of each solution and inject into high performance liquid chromatography Instrument, record chromatogram; With injection concentration (mg / mL) as X, conivaptan hydrochloride peak area (A) as Y, make linear regression equation, result is as follows table 2. Figure 4 Conivaptan hydrochloride standard curve.
[0078] Table 2 Preparation of Conivaptan Hydrochloride Content Determination Standard Curve
[0079...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com