An ion chromatography method for the simultaneous determination of main component mepiperium and its impurity n-methylpiperidine in pesticides
A technology of methylpiperidine and methylpiperium, applied in the field of analytical chemistry, can solve the problems of inability to use liquid chromatography ultraviolet detectors for detection, distinction, and inability to achieve qualitative analysis, and achieves the effect of meeting process control requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: Optimization of chromatographic conditions
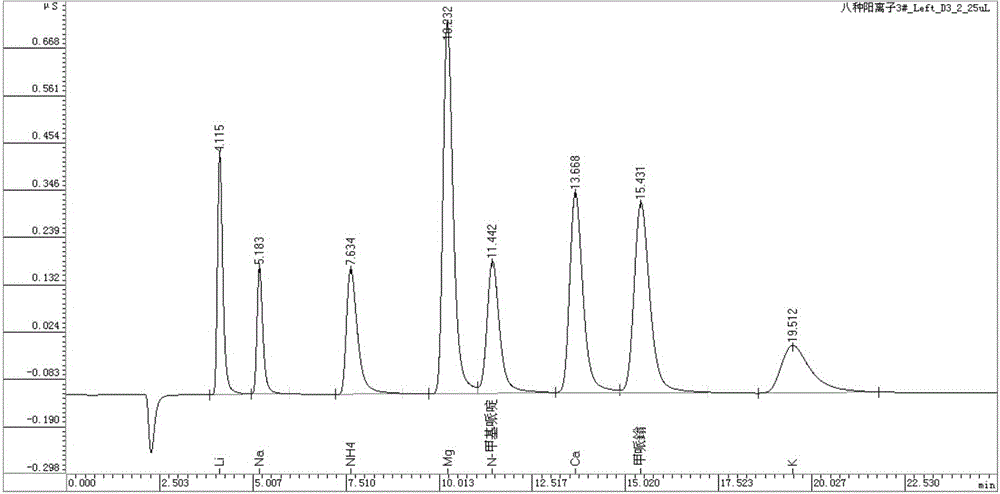
[0035] When a single 2mmol / L methanesulfonic acid solution was used as the eluent, it was found that N-methylpiperidine and Mg 2+ , Ca 2+ Inseparable from mepiperium, which cannot be resolved by increasing or decreasing the concentration of the methanesulfonic acid solution. Therefore, it is necessary to add an organic solvent to adjust the interaction between N-methylpiperidine and mepiperium and the chromatographic column filler, so as to realize that N-methylpiperidine and Mg 2+ , Ca 2+ Separation from methylpiperium. It is found through experiments that after adding 1.0mmol / L crown ether in the 2mmol / L methanesulfonic acid eluent, N-methylpiperidine and Mg 2+ , Ca 2+ Baseline separated from mepiperium, see figure 1 .
Embodiment 2
[0036] Embodiment 2: Analysis of N-methylpiperidine and mepiperium mixed standard solution
[0037] (1) Repeatability
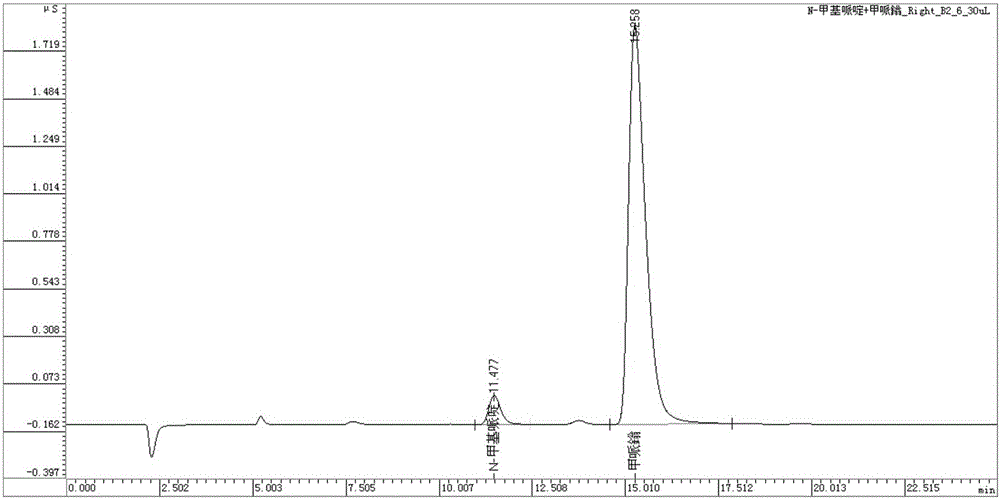
[0038] The standard mixed solution of the same concentration was continuously injected 6 times to obtain the chromatograms of N-methylpiperidine and mepiperium (see figure 2 ), and the relative standard deviations of their peak areas were 0.81% and 0.80%, respectively.
[0039] (2) Linear relationship
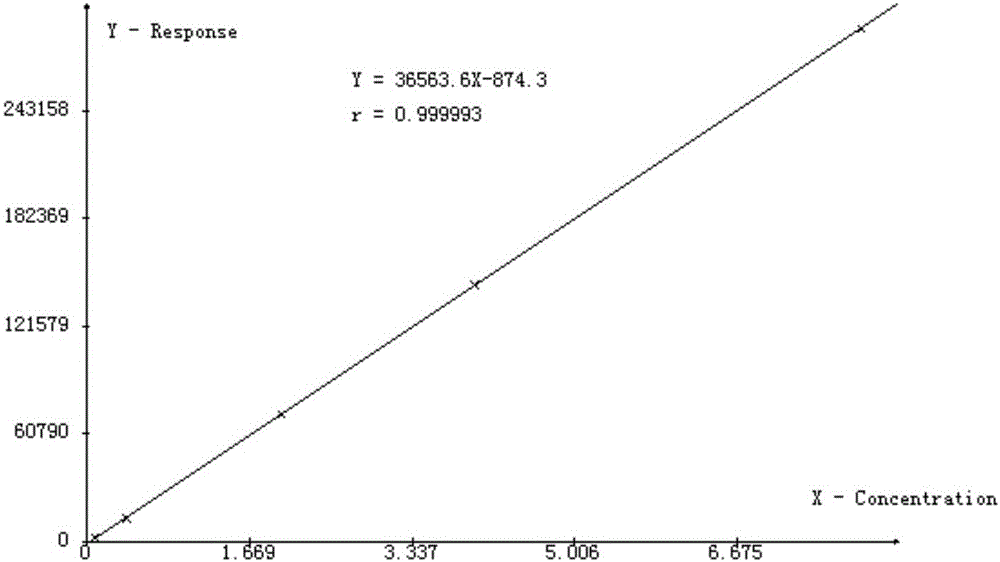
[0040] For the prepared linear solution of N-methylpiperidine and mepiperium, perform injection analysis according to the injection sequence from low concentration to high concentration, and obtain the peak areas of N-methylpiperidine and mepiperium at different concentrations , taking the concentration (mg / L) of the ion to be measured as the abscissa respectively, and taking its peak area as the ordinate to plot, obtain the working curve of N-methylpiperidine and mepiperium, attach respectively image 3 with Figure 4 .
Embodiment 3
[0041] Embodiment 3: the mensuration of the content of N-methylpiperidine and mepiperium in the pesticide
[0042] Under the selected chromatographic conditions, the actual sample is detected to obtain the chromatogram of the simultaneous detection of N-methylpiperidine and mepiperium, see the attached Figure 5 , N-methylpiperidine and mepiperium in the actual sample were quantified according to the external standard method, and the contents of N-methylpiperidine and mepiperium in the pesticide were obtained, as shown in Table 1. In the sample, N-methylpiperidine and mepiperium-0.1 and 5.0mg / L standard solutions were added respectively, and the recovery rate test was carried out by the method of adding standard. The recovery rate results are attached in Table 1.
[0043] The content detection result of table 1N-methylpiperidine and mepiperium
[0044]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com