Argatroban intermediate as well as preparation method and application thereof
A technology for argatroban and intermediates, which is applied in the field of argatroban intermediates and their preparation, and can solve the problems of difficult solids, many impurities, affecting intermediate storage and quality control, and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Embodiment 1: the synthesis of compound 3
[0065]
[0066] At -20°C, 9.0 g (88.9 mmol) of triethylamine and 12.2 g (89.3 mmol) of isobutyl chloroformate were added to a solution containing 36.2 g (88.6 mmol) of compound 1 in anhydrous tetrahydrofuran (450 ml) to keep - After stirring at 20°C for 10 minutes at 300 rpm, 10.6 g (62.0 mmol) of compound 2 was added thereto. After stirring at -20°C for 10 minutes, it was raised to room temperature within 1 hour and reacted for 16 hours. TLC (petroleum ether / ethyl acetate=10 / 1) Monitor the completion of the reaction, spin off the solvent tetrahydrofuran, then add ethyl acetate to it, successively use 5% (w / w) sodium bicarbonate solution, 10% (w / w) citric acid and saturated saline After washing, the organic phase was dried with anhydrous sodium sulfate, filtered, and the filtrate was spin-dried to obtain 44.1 g of compound 3 with a yield of 88.7% and a purity of 98.3%.
Embodiment 2
[0068] Embodiment 2: the synthesis of compound 4
[0069]
[0070] Under an ice-water bath, add 200mL of 4mol / L methanolic hydrogen chloride solution to the methanol solution (100ml) containing 40g (71.3mmol) of compound 3, control the internal temperature below 20°C, stir at 300rpm for 3 hours, then add 10% hydrogen Sodium oxide aqueous solution was 35mL, and then 200mL of methyl tert-butyl ether was added, a solid was formed, the stirring was continued for half an hour, filtered, the filter cake was washed and dried with methyl tert-butyl ether to obtain 27.9g of compound 4, yield: 85.2%, Purity: 97.4%.
[0071] The mass spectrum NMR data of compound 4 is: ESI-MS (m / z): 462.3[M+H] + ;1H NMR (400MHz, DMSO-d6)δ:8.05(s,2H),7.40(s,1H),7.34(m,4H),7.32(dd,1H),5.04(s,2H),4.46(m ,1H),4.14(m,3H),3.42(m,4H),2.44(s,1H),2.08(s,1H),2.03(m,2H),1.74(m,4H),1.64(m, 3H), 1.22(t,3H), 0.86(d,3H).
Embodiment 3
[0072] Embodiment 3: the synthesis of compound 6
[0073]
[0074] At 0°C, add 18.5g (183mmol) triethylamine to a solution containing 27.4g (59.5mmol) of compound 4 in dichloromethane (200ml), then add 14.7g (60.8mmol) of compound 5 to it, and heat up to 75 °C for more than 5 hours, followed by TLC until the reaction was complete.
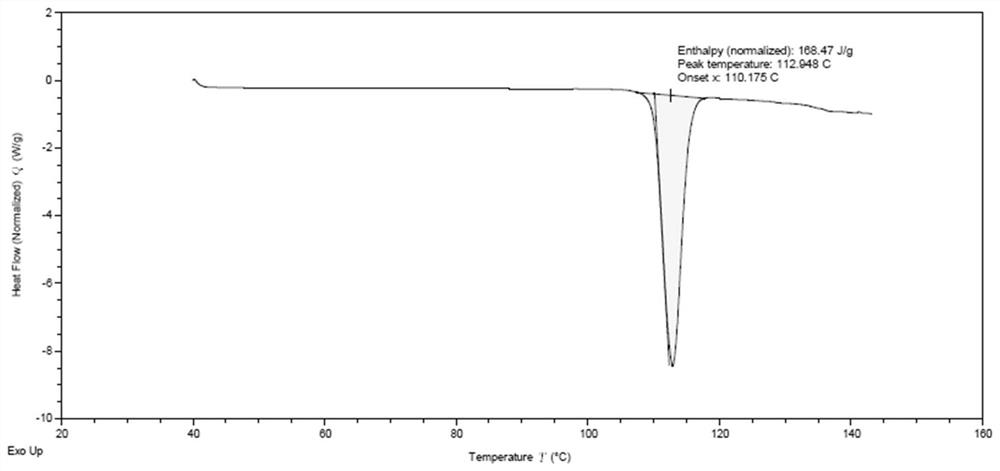
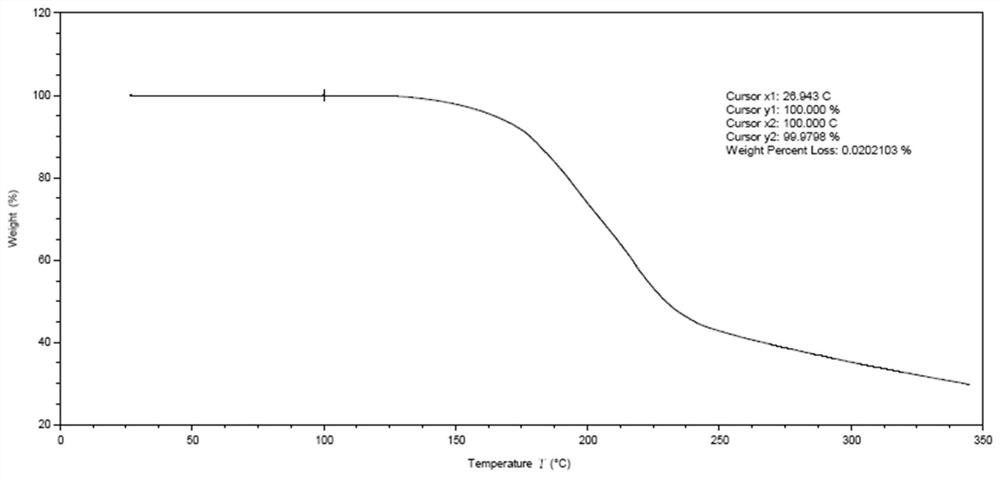
[0075] The reaction solution was washed twice with water, the organic phase was dried with anhydrous sodium sulfate, the solvent was evaporated to dryness and then crystallized with 30% (v / v) acetonitrile aqueous solution to obtain 34.4g of compound 6 with a yield of 86.7% and a purity of 98.8%. from figure 1 and figure 2 It can be seen that compound 6 is a better solid.
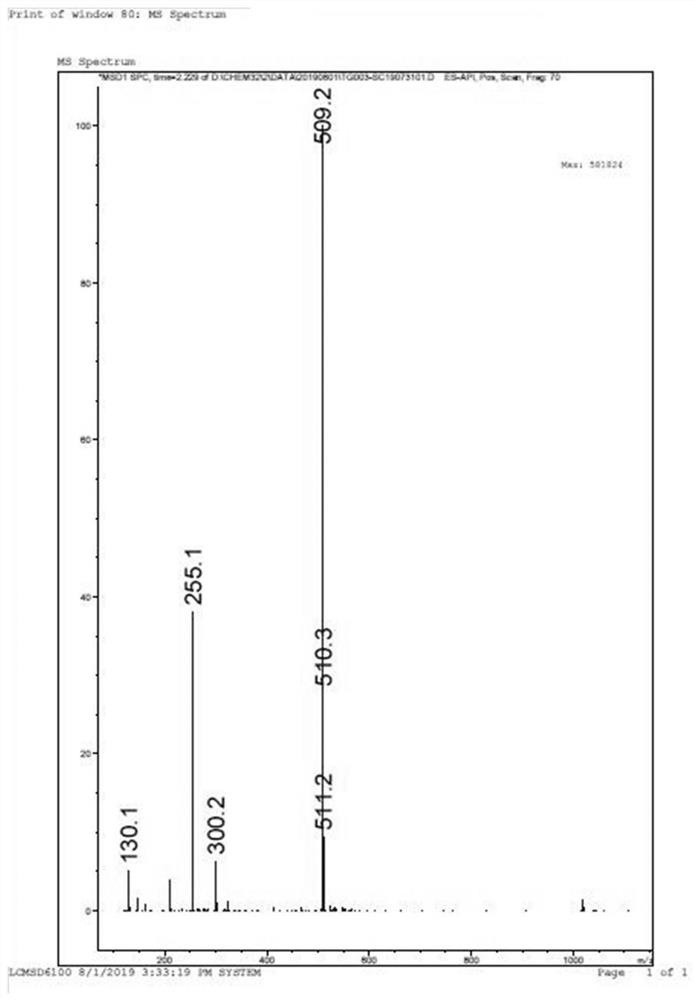
[0076] The mass spectrum NMR data of compound 6 is: ESI-MS (m / z): 667.3[M+H] + ;1H NMR (400MHz, DMSO-d6)δ:8.92(d,1H),8.48(s,1H),8.20(m,3H),7.70(m,1H),7.33(m,4H),7.32(m ,2H),5.02(s,2H),4.78(s,1H),4.58(s,1H),4.35(s,1H),4.05(m,1H),3.92(m,1H),3.69(m, 1H), 3.03(d, 3H), 2.54(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com