Solid-state electrolyte material of lithium ion battery
A solid-state electrolyte, lithium-ion battery technology, used in secondary batteries, circuits, electrical components, etc., can solve the problems of long cycle, unclear microscopic action mechanism, and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
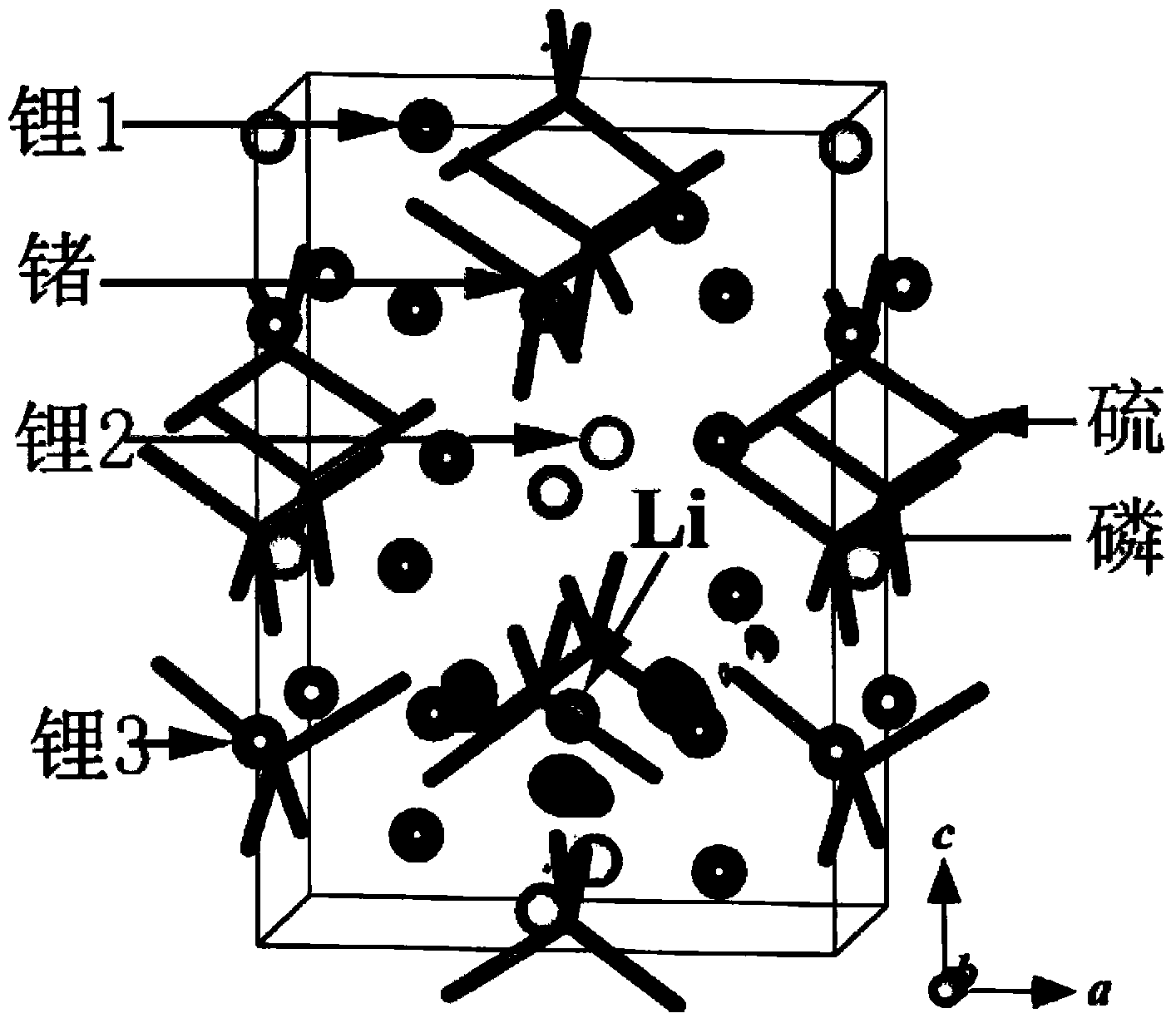
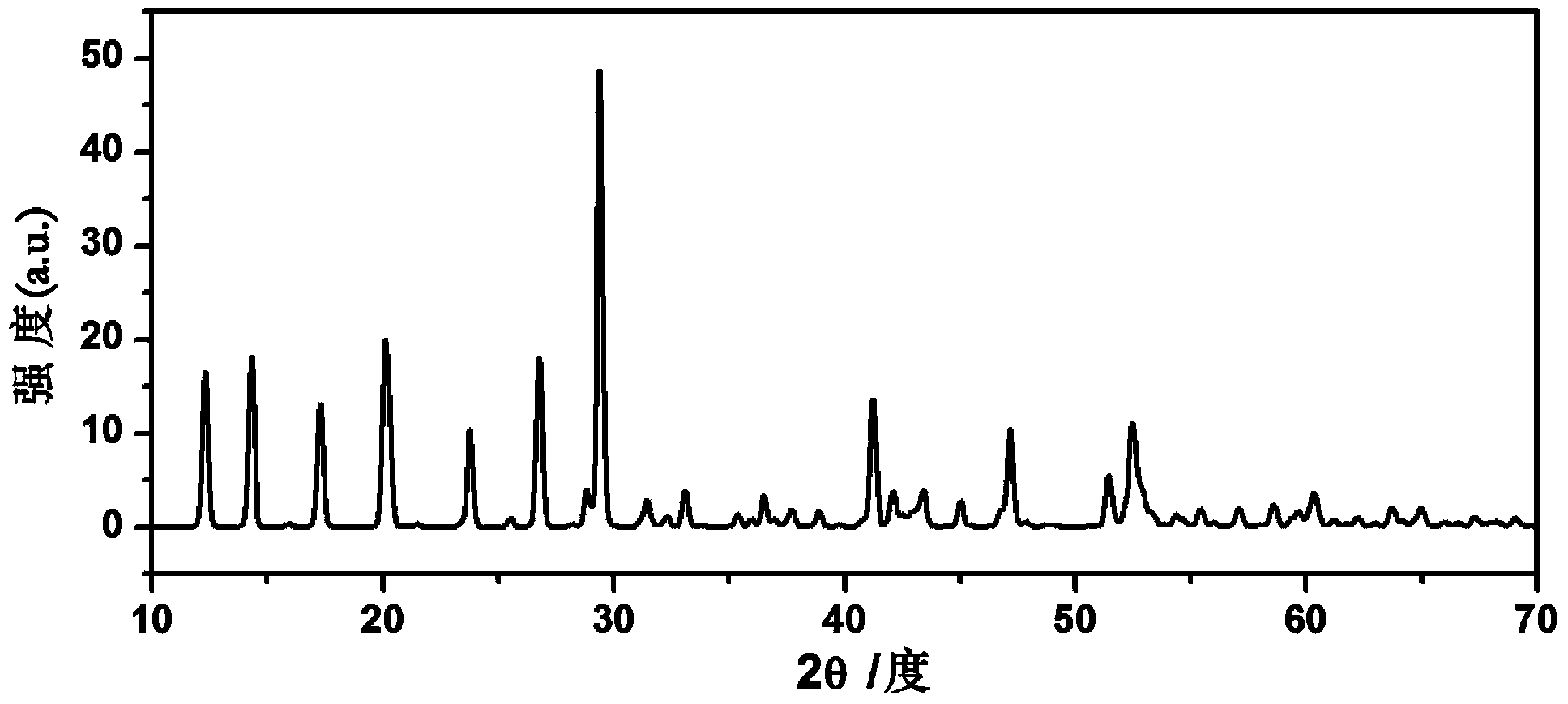
[0032] Li 2 S, CaS, P 2 S 5 with GeS 2 According to the molar ratio of 4:1:1:1, it was ball-milled in a high-energy ball mill until uniform, and the ball-milled powder was obtained; then the ball-milled powder was placed in a quartz tube filled with argon, and treated at 600 ° C for 8 hours to obtain Li 8 CaP 2 S 12 Crystalline particles, then cooled slowly to room temperature. Solid electrolyte material Li 8 CaP 2 S 12 Doped with Ca 2+ . The doped Ca 2+ relative to Li + Available with surrounding S 2- form a stronger Coulomb attraction, and can shield S more strongly 2- with S 2- Coulomb repulsion between . Ca 2+ Doped with Li + before and after, S 2- from 2+ The number of charges obtained around is significantly higher than that obtained from Li + The number of charges obtained around, that is, Ca 2+ with S 2- The Coulomb attraction between Li + with S 2- The Coulomb attraction between is stronger, while S 2- The Coulomb repulsion between can also b...
Embodiment 2
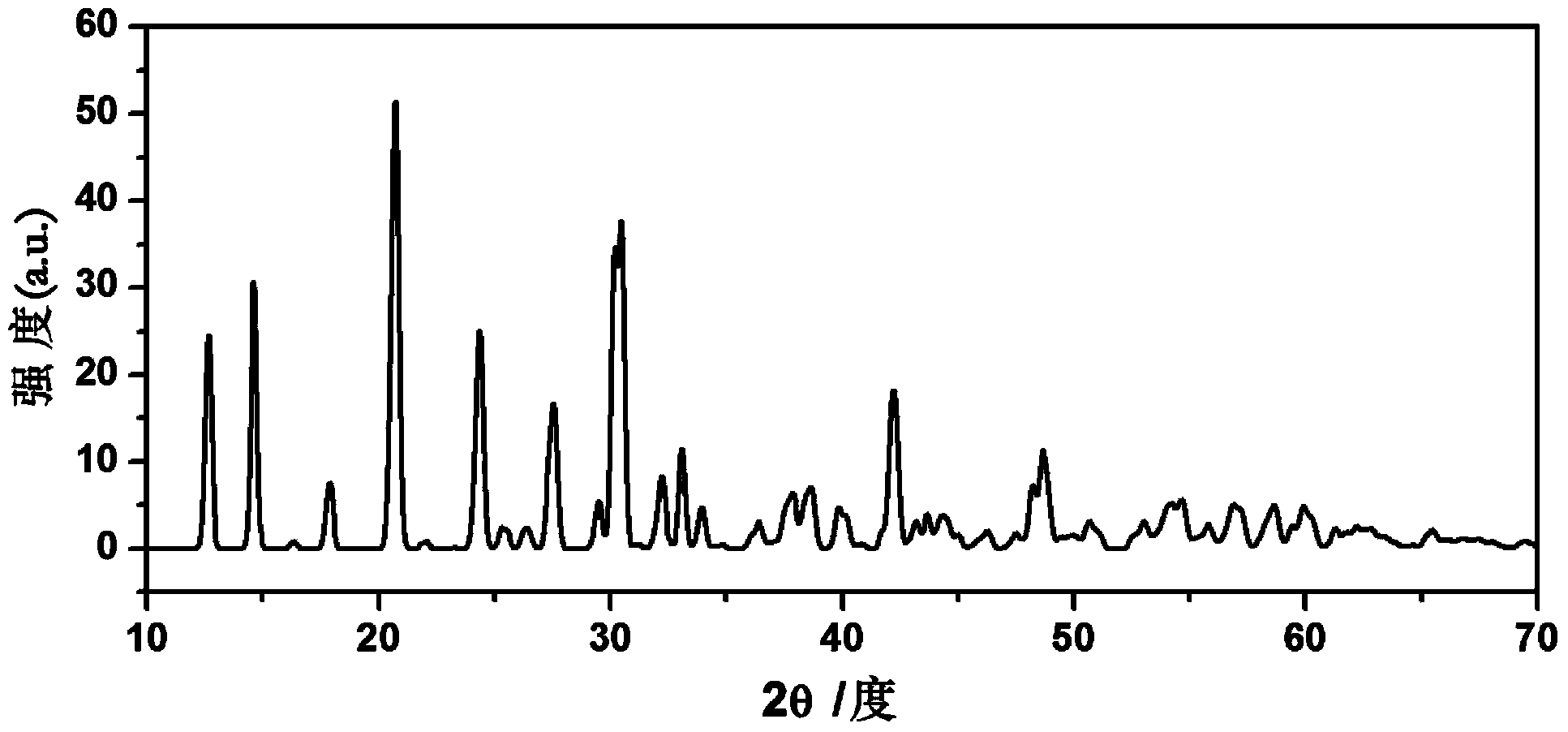
[0034] Li 2 S, CaS, P 2 S 5 with GeS 2 According to the molar ratio of 4.5:0.5:1:1, it was ball-milled in a high-energy ball mill until uniform, and the ball-milled powder was obtained; then the ball-milled powder was placed in a quartz tube filled with argon, and treated at 600 ° C for 8 hours to obtain Li 9 Ca 0.5 GeP 2 S 12 Crystalline particles, then cooled slowly to room temperature. Solid electrolyte material Li 9 Ca 0.5 GeP 2 S 12 Doped with Ca 2+ . The doped Ca 2+ relative to Li + Available with surrounding S 2- form a stronger Coulomb attraction, and can shield S more strongly 2- with S 2- Coulomb repulsion between . Ca 2+ Doped with Li + before and after, S 2- from 2+ The number of charges obtained around is significantly higher than that obtained from Li + The resulting number of charges around (see Figure 4 and figure 1 contrast), that is, Ca 2+ with S 2- The Coulomb attraction between Li + with S 2- The Coulomb attraction between is ...
Embodiment 3
[0036] Li 2 S, CaS, P 2 S 5 with Al 2 S 3 According to the molar ratio of 4.5:1:1:0.5, it was ball-milled in a high-energy ball mill until uniform, and the ball-milled powder was obtained; then the ball-milled powder was placed in a quartz tube filled with argon, and treated at 600 ° C for 8 hours to obtain Li 9 CaAlP 2 S 12 Crystalline particles, then cooled slowly to room temperature. Solid electrolyte material Li 9 CaAlP 2 S 12 Doped with Ca 2+ . The doped Ca 2+ relative to Li + Available with surrounding S 2- form a stronger Coulomb attraction, and can shield S more strongly 2- with S 2- Coulomb repulsion between . Ca 2+ Doped with Li + before and after, S 2- from 2+ The number of charges obtained around is significantly higher than that obtained from Li + The number of charges obtained around, that is, Ca 2+ with S 2- The Coulomb attraction between Li + with S 2- The Coulomb attraction between 2- The Coulomb repulsion between can also be better ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com