Fluorescence color-change material capable of directionally identifying dichloromethane, trichloromethane and tetrachloromethane and preparation method of fluorescence color-change material
A color-changing material, fluorescence technology, applied in the intersection of nanomaterials and coordination chemistry, to achieve the effect of high yield, good stability and fast response speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: Synthesis of organic solvent-induced fluorescent color-changing materials
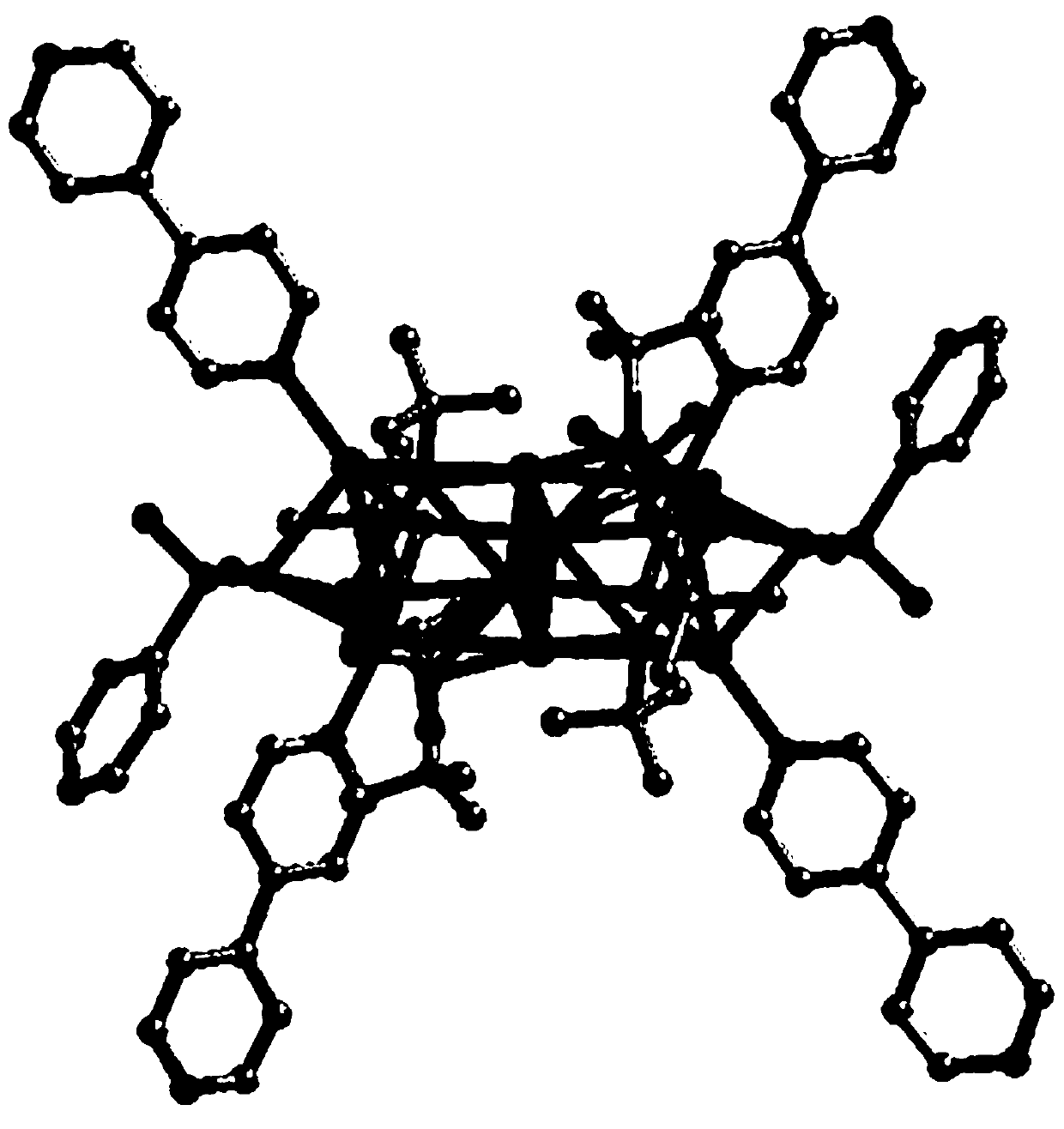
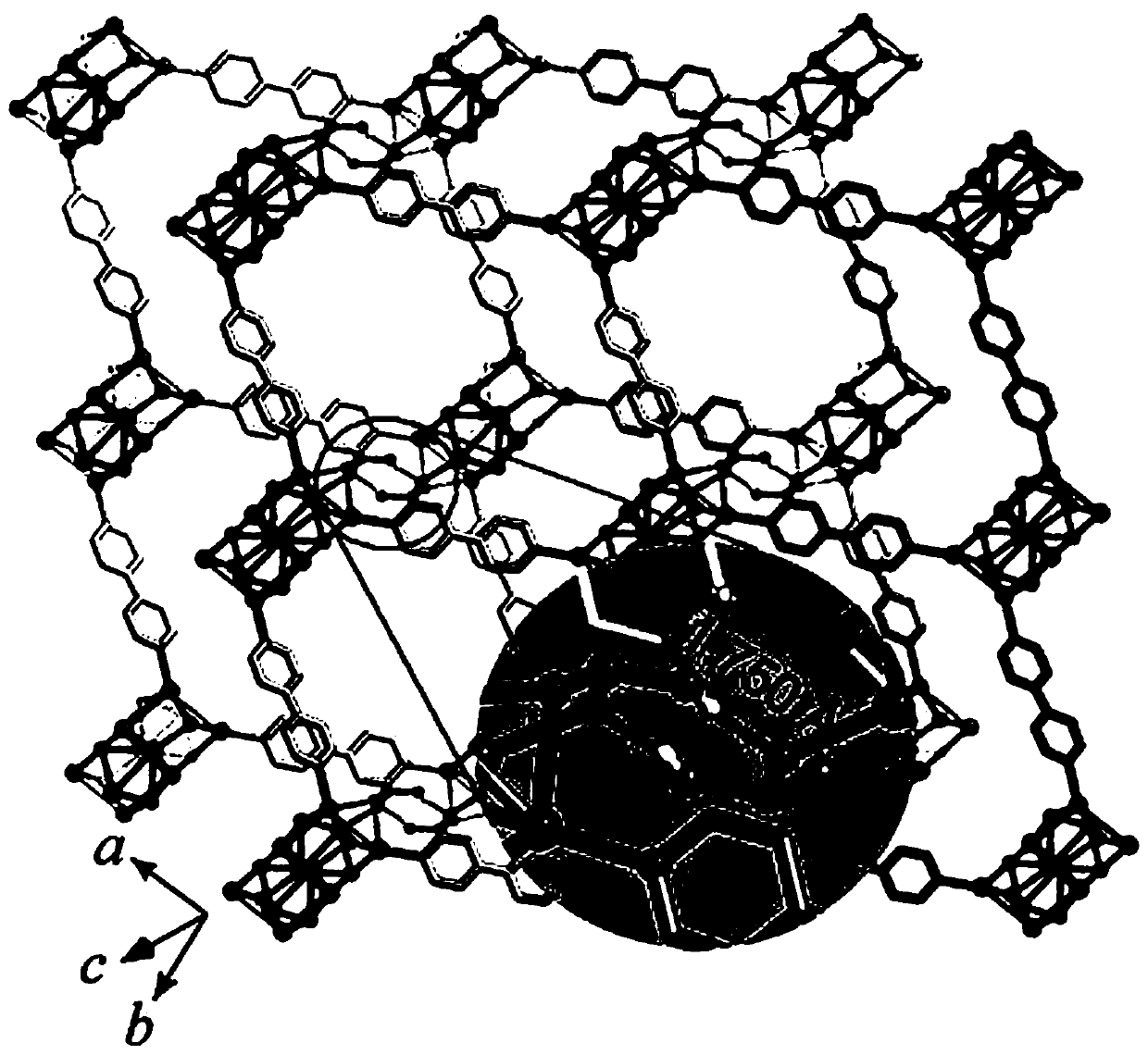
[0027] 0.02g (0.1mmol) silver tert-butylsulfide (Bu t SAg) is placed in the mixed solution of 3mL acetone and 3mL ethanol, stir rapidly; Add 0.02g (0.1mmol) silver trifluoroacetate (CF 3 COOAg), stirred until the solution was clear; 0.02 g (0.13 mmol) of phenylphosphonic acid was added, and finally 0.02 g (0.12 mmol) of 4,4'-bipyridine (bpy) was added, and stirring was continued for 5 minutes. After the reaction, the solution was evaporated at room temperature in the dark, and after 3 days, about 20 mg of light yellow blocky crystals were obtained, with a yield of 75%. After filtering, washing with ethanol, and drying at room temperature, an organic solvent-induced fluorescent color-changing material was obtained.
Embodiment 2
[0028] Embodiment 2: The material of the present invention recognizes and detects the phenomenon of organic solvents
[0029] Get the organic solvent-induced fluorescent color-changing material sample prepared in Example 1, under the irradiation of a 365nm ultraviolet lamp, drop CH on the material respectively 2Cl 2 , CHCl 3 , CCl 4 , the fluorescent colors of the materials are green, orange, and yellow-green, respectively. From Figure 4 and 5 It can be seen that the material realizes the detection of these three solvents and can be identified by naked eyes.
Embodiment 3
[0030] Embodiment 3: Fluorescence spectrometry and solvent adsorption test for organic solvent identification of the material of the present invention.
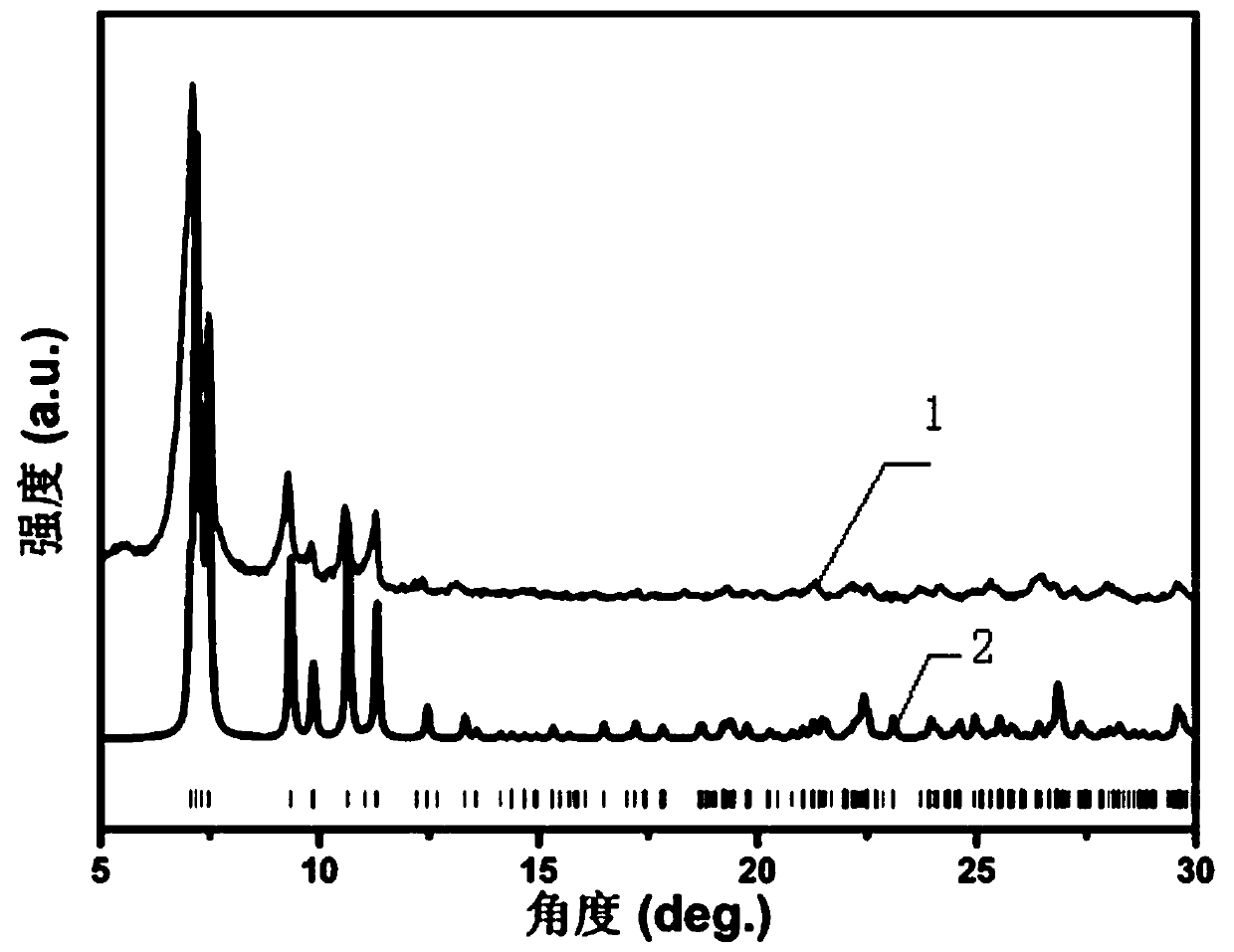
[0031] Get 20mg of the organic solvent-induced fluorescent color-changing material powder sample that embodiment 1 makes, pack in the fluorescent test solid sample tank, drop 25 μ L organic solvent (CH 2 Cl 2 , CHCl 3 , CCl 4 , wet the material completely), measure its fluorescence spectrum (excitation wavelength 365nm). The fluorescence maximum emission peak wavelength of the material of the present invention is different under different organic solvents. Compared with dichloromethane, chloroform and carbon tetrachloride have a better stabilizing effect on the excited state of the material, so the fluorescence emission position is red-shifted. Under the action of methyl chloride, the fluorescence emission position of the material does not change compared with its own fluorescence emission position. After the organic solv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com