Method for preparing iloperidone
A technology of iloperidone and ethyl ketone, applied in the field of preparation of iloperidone, can solve the problem of affecting the efficacy of iloperidone preparations, affecting the yield and purity of iloperidone, and being difficult to remove by-products, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
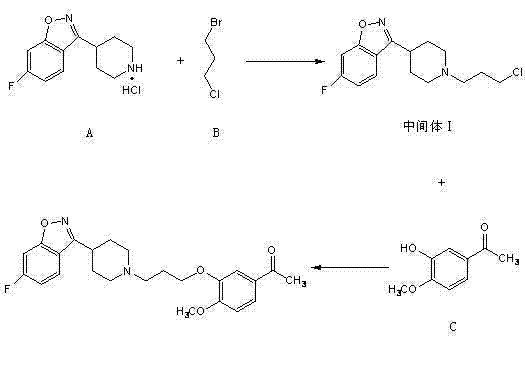
[0021] (1) Preparation of 3-[1-(3-chloropropyl)piperidin-4-yl]-6-fluoro-1,2-phenylpropisoxazole
[0022] Mix 68g of 6-fluoro-3-(4-piperidinyl)-1,2-benzisoxazole hydrochloride, 22g of sodium hydroxide and 400ml of acetonitrile completely, and add 54g of 1- Bromo-3-chloropropane and 40ml of acetonitrile were incubated and stirred for 12 hours, then the mixture was poured into water at 0°C with stirring, filtered to obtain a solid, and dried to obtain 70g of 3-[1-(3-chloropropyl)piperidine -4-yl]-6-fluoro-1,2-phenylisoxazole, yield 89.1%;
[0023] (2) Preparation of iloperidone
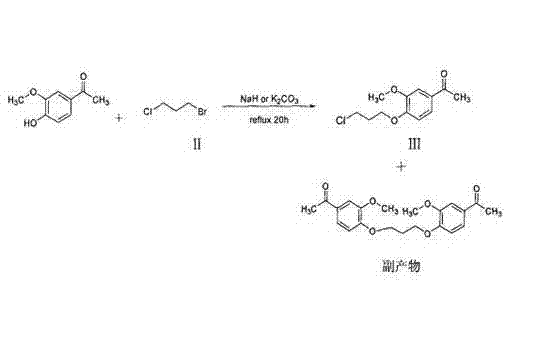
[0024] 70g 3-[1-(3-chloropropyl)piperidin-4-yl]-6-fluoro-1,2-phenylpropisoxazole, 37.5g sodium carbonate and 600ml isopropanol were mixed completely, at 40 At ℃, add 9g of potassium iodide, 39g of 4-hydroxy-3-methoxyacetophenone and 100ml of isopropanol, react at 40℃ for 35 hours, filter, and concentrate the filtrate to dryness to obtain a semi-solid, which Add 175ml of methanol, heat to dissolve comp...
Embodiment 2
[0027] (1) Preparation of 3-[1-(3-chloropropyl)piperidin-4-yl]-6-fluoro-1,2-phenylpropisoxazole
[0028] 677g 6-fluoro-3-(4-piperidinyl)-1,2-benzisoxazole hydrochloride, 360g potassium hydroxide and 1200ml N,N-dimethylformamide, 1200ml N,N- Mix dimethylacetamide and 1600ml dimethylsulfoxide completely, add 538g 1-bromo-3-chloropropane and 150ml N,N-dimethylformamide, 150ml N,N-di Methyl acetamide, 100ml dimethyl sulfoxide, heat preservation and stirring for 12 hours, then pour the mixture into water at 5°C with stirring, filter to obtain a solid, dry to obtain 3-[1-(3-chloropropyl)piperene Pyridin-4-yl]-6-fluoro-1,2-phenylisoxazole 708g, yield 90.5%;
[0029] (2) Preparation of iloperidone
[0030] 708g 3-[1-(3-chloropropyl)piperidin-4-yl]-6-fluoro-1,2-phenylpropisoxazole, 98g sodium hydroxide and 3000ml 1,4-dioxane , 3000ml tetrahydrofuran and mixed completely, at 60°C, add 88g sodium iodide, 396g 4-hydroxy-3-methoxyacetophenone and 500ml 1,4-dioxane, 500ml tetrahydrofuran...
Embodiment 3
[0033] (1) Preparation of 3-[1-(3-chloropropyl)piperidin-4-yl]-6-fluoro-1,2-phenylpropisoxazole
[0034]331g of 6-fluoro-3-(4-piperidinyl)-1,2-benzisoxazole hydrochloride, 171g of sodium ethylate and 1200ml of N,N-dimethylacetamide, 800ml of acetonitrile were mixed completely, in At 5-10°C, add 268g of 1-bromo-3-chloropropane, 120ml of N,N-dimethylacetamide, and 80ml of acetonitrile, keep stirring for 8 hours, then pour the mixture into water at 0°C while stirring, and filter. The solid was obtained and dried to obtain 338g of 3-[1-(3-chloropropyl)piperidin-4-yl]-6-fluoro-1,2-phenylpropisoxazole with a yield of 88.5%;
[0035] (2) Preparation of iloperidone
[0036] 338g 3-[1-(3-chloropropyl)piperidin-4-yl]-6-fluoro-1,2-phenylpropisoxazole, 181.2g potassium tert-butoxide and 2900ml acetone were mixed completely, at 50 At ℃, add 44g of potassium iodide, 190g of 4-hydroxy-3-methoxyacetophenone and 500ml of acetone, react at 50℃ for 30 hours, filter, and concentrate the filtrat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com