Iloperidone pharmaceutical composition and preparation method thereof
A technology of iloperidone and composition, which is applied in the field of iloperidone pharmaceutical composition and its preparation, and can solve problems such as not giving further prompts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
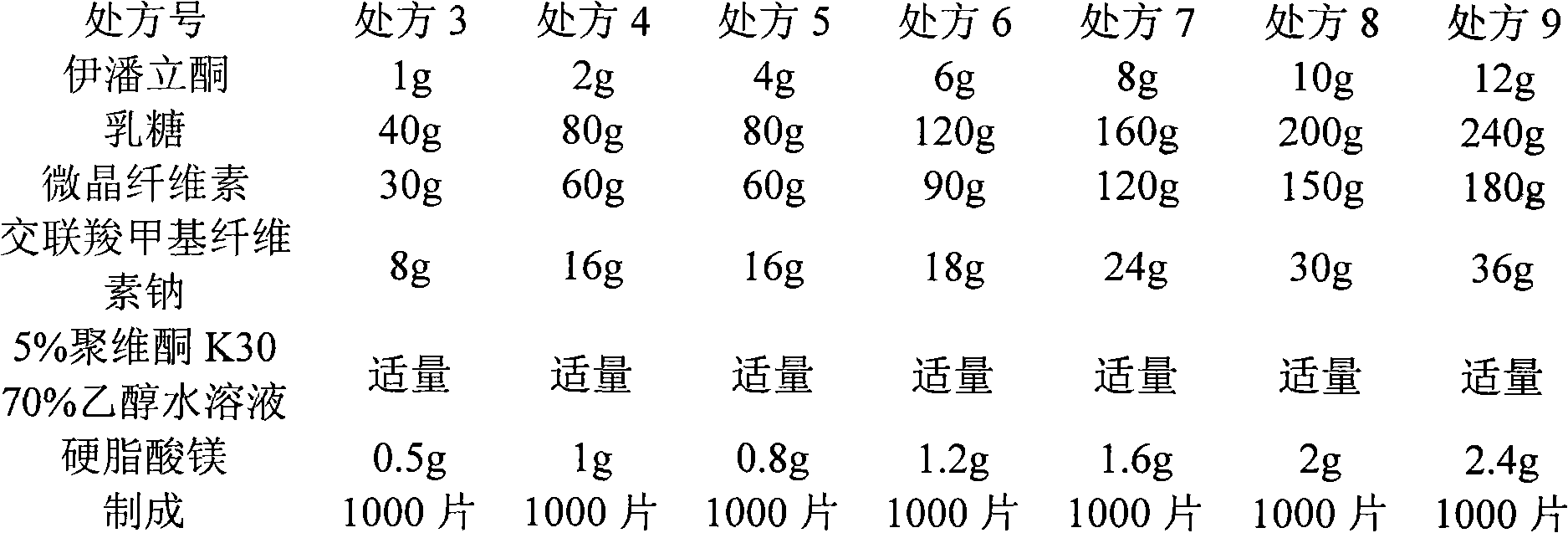
[0132] Tablet prescription composition, each 1000 tablets are composed as follows:
[0133] Iloperidone 1g
[0134] Lactose 40g
[0135] Microcrystalline Cellulose 30g
[0136] Croscarmellose Sodium 8g
[0137] Magnesium stearate 0.5g
[0138] 5% povidone K30 70% ethanol solution appropriate amount
[0139] Preparation Process
[0140] 1) Grinding the iloperidone until the average particle size is less than 80 μm.
[0141] 2) Pass lactose, microcrystalline cellulose, and croscarmellose sodium magnesium stearate through an 80-mesh sieve for subsequent use.
[0142] 3) According to the prescription quantity, the iloperidone, lactose, microcrystalline cellulose, croscarmellose sodium, and magnesium stearate of the prescription quantity were weighed after double-checking.
[0143] 4) Mix iloperidone and other excipients except magnesium stearate uniformly in equal increments.
[0144] 5) Use 5% povidone K3070% ethanol solution to make soft material, and granulate with 16-3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com