Iloperidone composition and preparation method thereof
A technology for iloperidone and a composition is applied in the field of pharmaceutical compositions containing iloperidone and the preparation thereof, and can solve the problems of poor fluidity, large difference in tablet weight, loose particles, etc. Simple composition, rapid dissolution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
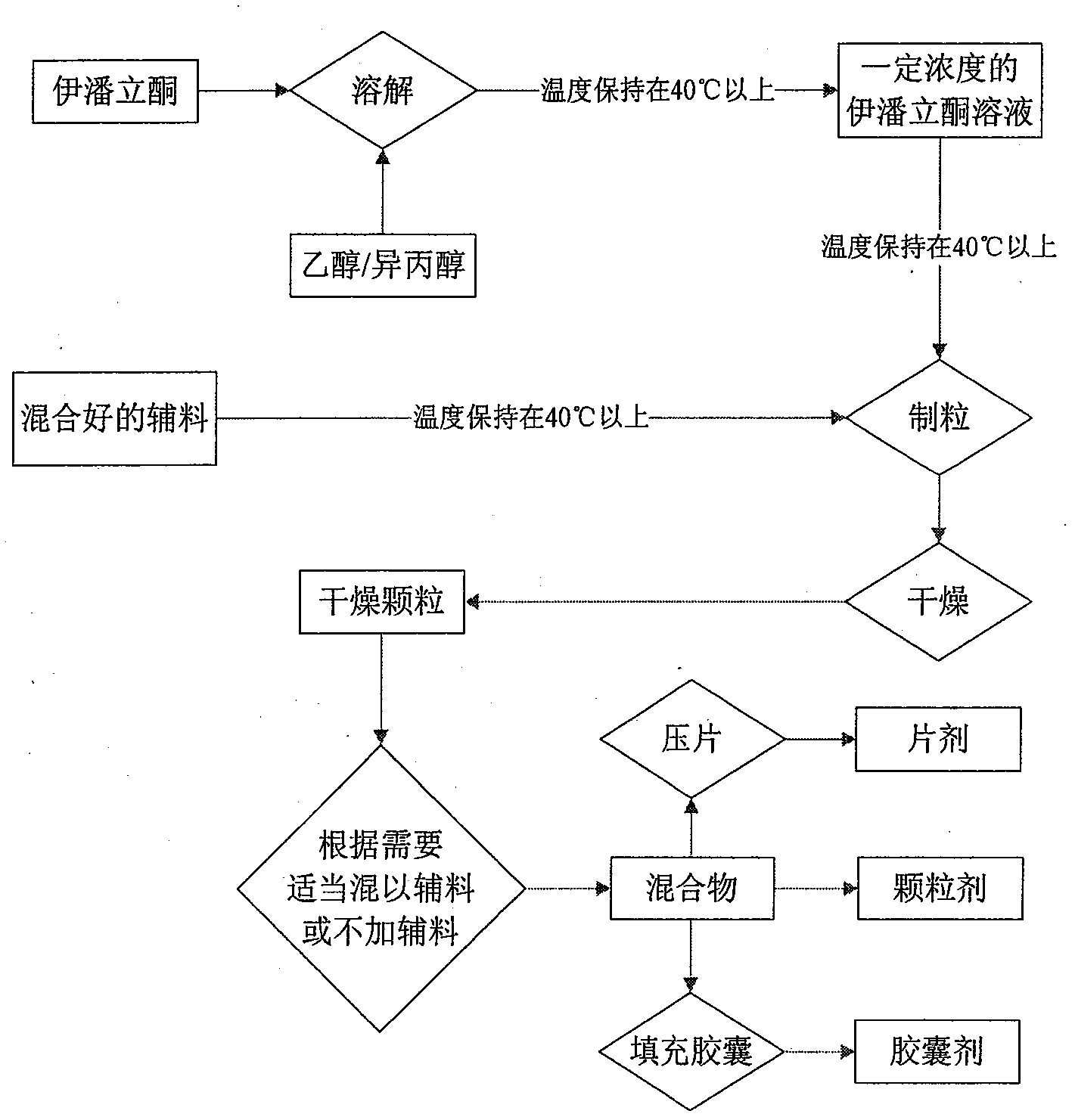
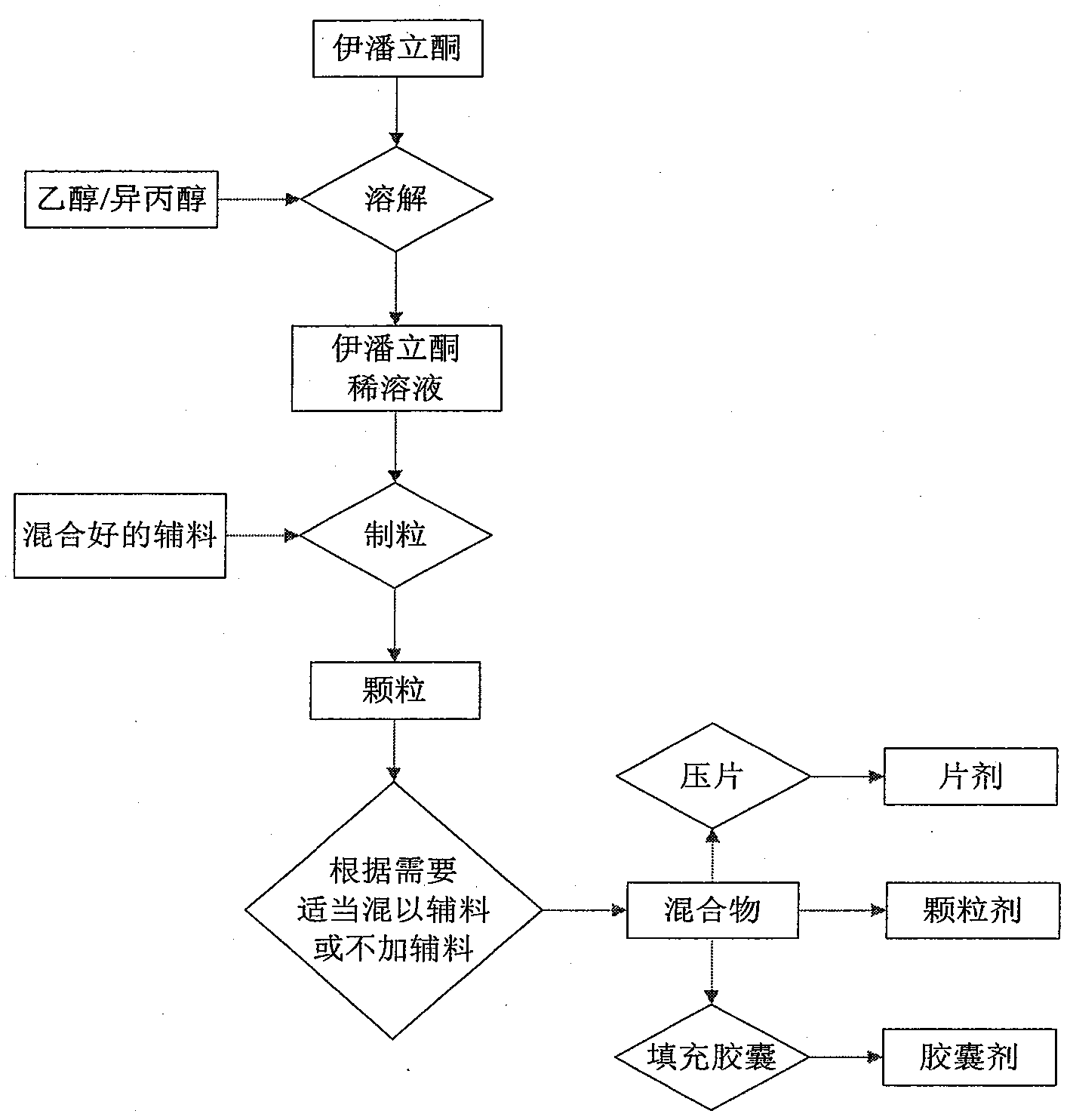
Examples
Embodiment 1
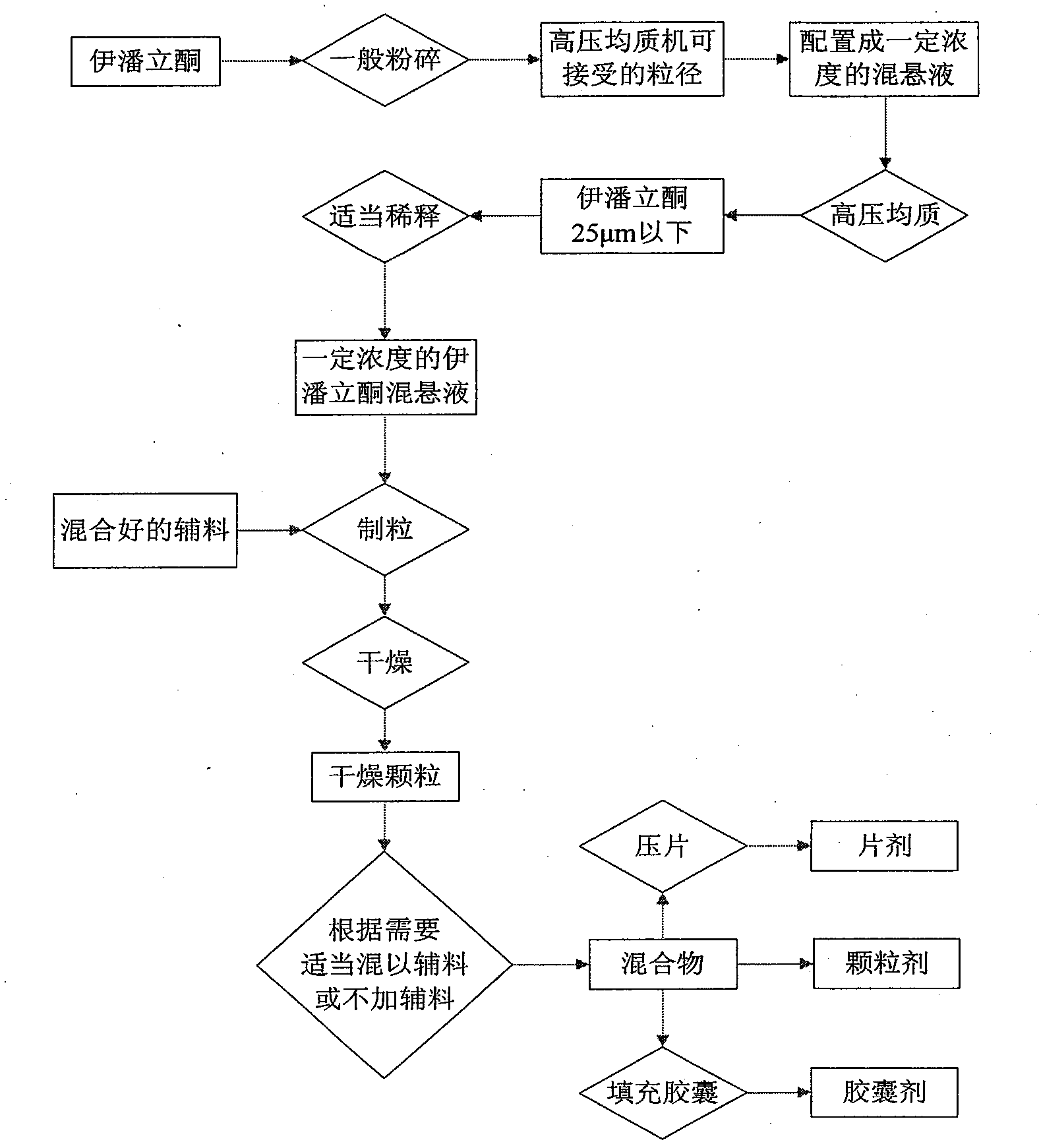
[0079] Example 1 High-pressure homogenization method, controlling more than 90% of the particle size to be less than 5 μm
[0080]
[0081]
[0082] Preparation Process:
[0083] i) The iloperidone raw material is pulverized and passed through a 100-mesh sieve (150 μm) for subsequent use;
[0084] ii) Disperse iloperidone passing through a 100 mesh sieve in 2.5% hydroxypropyl methylcellulose aqueous solution to form an initial suspension containing 13% iloperidone;
[0085] iii) The primary suspension is homogenized under high pressure until the particle size of iloperidone in the suspension is more than 90% and less than 5 μm;
[0086] iv) measure iloperidone content in the suspension after high-pressure homogenization, calculate, and dilute to the suspension that iloperidone content is 10% with 2.5% hydroxypropyl methylcellulose aqueous solution;
[0087] v) Weigh lactose, microcrystalline cellulose, 6% crospovidone and hydroxypropyl methylcellulose powder that has ...
Embodiment 2
[0092] Embodiment 2 High-pressure homogeneous method, controlling more than 90% of the particle size to be less than 10 μm
[0093]
[0094]
[0095] Preparation Process:
[0096] i) The iloperidone raw material is pulverized and passed through a 100-mesh sieve (150 μm) for subsequent use;
[0097] ii) Disperse iloperidone passing through a 100 mesh sieve in 2.5% hydroxypropyl methylcellulose aqueous solution to form an initial suspension containing 13% iloperidone;
[0098] iii) The initial suspension is homogenized under high pressure until the particle size of iloperidone in the suspension is more than 90% and less than 10 μm;
[0099] iv) measure iloperidone content in the suspension after high-pressure homogenization, calculate, and dilute to the suspension that iloperidone content is 10% with 2.5% hydroxypropyl methylcellulose aqueous solution;
[0100] v) Weigh lactose, microcrystalline cellulose, crospovidone and hydroxypropyl methylcellulose powder not config...
Embodiment 4
[0107] Embodiment 4 High-pressure homogenization method, controlling more than 90% of the particle size to be less than 25 μm
[0108]
[0109] Preparation Process:
[0110] i) The iloperidone raw material is pulverized and passed through a 100-mesh sieve (150 μm) for subsequent use;
[0111] ii) Disperse iloperidone passing through a 100 mesh sieve in 5% hydroxypropyl methylcellulose aqueous solution to form an initial suspension containing 21% iloperidone;
[0112] iii) The primary suspension is homogenized under high pressure until the particle size of iloperidone in the suspension is more than 90% and less than 25 μm;
[0113] iv) measure the iloperidone content in the suspension after high-pressure homogenization, calculate, and dilute to the suspension that the iloperidone content is 20% with 5% hydroxypropyl methylcellulose aqueous solution;
[0114] v) Weigh lactose, microcrystalline cellulose, 6% crospovidone and hydroxypropyl methylcellulose powder that has not...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com