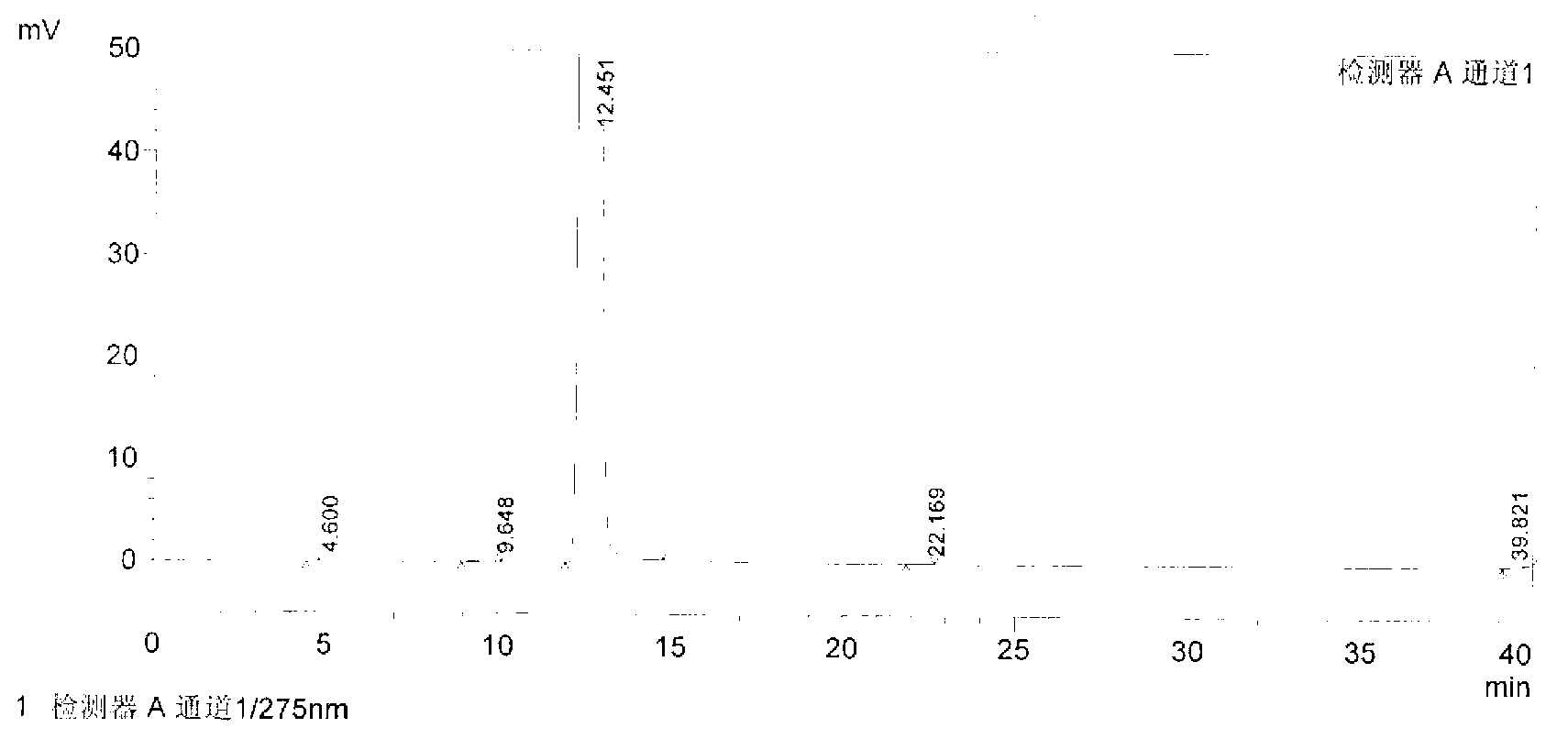
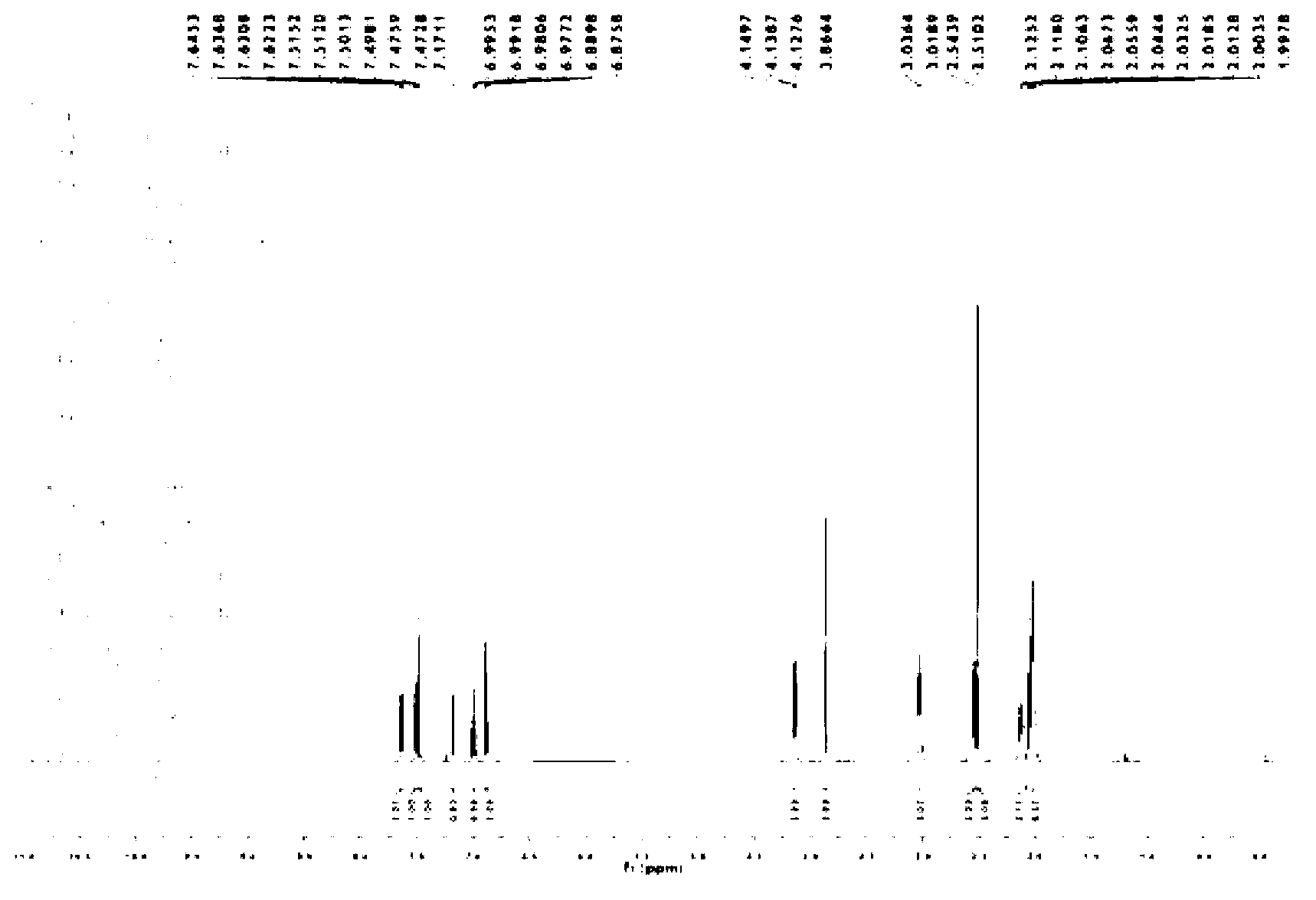
Method for preparing iloperidone
A technology of iloperidone and piperidinyl ketone oxime, which is applied in the field of pharmacy, can solve the problems of reduced yield and high toxicity of toluene, and achieve the effects of low cost, cheap reagents and low impurity content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]Example 1: Preparation of intermediate 2,4-difluorophenyl-4-piperidinyl ketone oxime (YPT-001)
[0030] Step 1: Add 4-(2,4-difluorobenzoyl)-piperidine hydrochloride (100g, 0.38mol) and hydroxylamine hydrochloride (100g, 1.44mol) into a 2000mL three-necked flask, add 1050mL95% ethanol, Stir, add triethylamine (88g, 0.87mol), heat to reflux for 4.5h, TLC shows that the reaction of raw materials is complete (developing solvent: DCM:MeOH=15:1 plus a little TEA). Cool to room temperature, filter with suction, wash with 500ml of ethanol, and dry under reduced pressure at 40°C to obtain about 67g of white solid with a yield of 73% and a purity of 91%.
Embodiment 2
[0031] Example 2: Preparation of intermediate 2,4-difluorophenyl-4-piperidinyl ketone oxime (YPT-001)
[0032] Step 1: Add 4-(2,4-difluorobenzoyl)-piperidine hydrochloride (15g, 0.057mol) and hydroxylamine hydrochloride (15g, 0.216mol) into a 250mL three-necked flask, add 160ml of 95% ethanol, Stir, add triethylamine (20ml, 0.144mol), heat to reflux for 3h, TLC shows that the reaction of raw materials is complete (developing solvent: DCM:MeOH=15:1 plus a little TEA). Cool to room temperature, filter with suction, wash with 80ml of ethanol, and dry to obtain 10.5g of white solid with a yield of 77% and a purity of 91%.
Embodiment 3
[0033] Example 3: Preparation of intermediate 6-fluoro-3-(4-piperidinyl)-1,2-benzisoxazole hydrochloride (YPT-002)
[0034] Step 2: Add potassium hydroxide (135.3g, 2.41mol) and 3.5L absolute ethanol into a 20L reactor, stir, and add the crude product 2,4-difluorophenyl-(4-piperidinyl)methanone oxime (290g, 1.21mol), react at a temperature of 40°C for 2.5h, TLC shows that the reaction of the raw material is complete (developer: DCM:MeOH=15:1 plus a little TEA), remove the oil bath, cool to room temperature, filter with suction, wash with a little ethanol filter cake. The filtrate was evaporated to dryness, the residue was added with about 5L of water, extracted with dichloromethane (4.5L to 2.5L), washed once with 5L of saturated saline, once with 5L of water, and dried over anhydrous sodium sulfate. Filter and evaporate the solvent under reduced pressure to obtain 200 g (0.908 mol) of the free 6-fluoro-3-(4-piperidinyl)-1,2-benzisoxazole crude product, add 2.2 L of methanol ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com