Preparation method of iloperidone
A technology of iloperidone and step method, applied in a new field of preparation, can solve the problems of a large number of solvents, cumbersome operation, and long reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 1, the preparation of 4-(3-chloropropyloxy)-3-methoxyacetophenone (i.e. formula 3 compound)
[0026] Dissolve 17.6 g of vanillone in 75 ml of acetone, add 13.8 g of potassium carbonate, heat to reflux, dropwise add a mixed solution of 83.4 g of 1-bromo-3-chloropropane and 20 ml of acetone, and react at reflux for 10 h. Cool to room temperature, filter with suction to obtain the filtrate, evaporate acetone and 1-bromo-3-chloropropane, and distill under reduced pressure to obtain 21.2 g of crude product of (3-chloropropyloxy)-3-methoxyacetophenone. The rate is 82.4%.
[0027] 2. Preparation of (Z)-4-(2,4-difluorophenyl)-piperidinyl ketone oxime (i.e. compound of formula 4)
[0028] Add 136ml of absolute ethanol and 11.2g of hydroxylamine hydrochloride into a 500ml three-necked flask, add 54.7g of 20% NaOH aqueous solution under stirring, and control the temperature at 20-30°C. After the dropwise addition is completed, stir for 15 minutes, and the pH is about 10. Then 2...
Embodiment 2
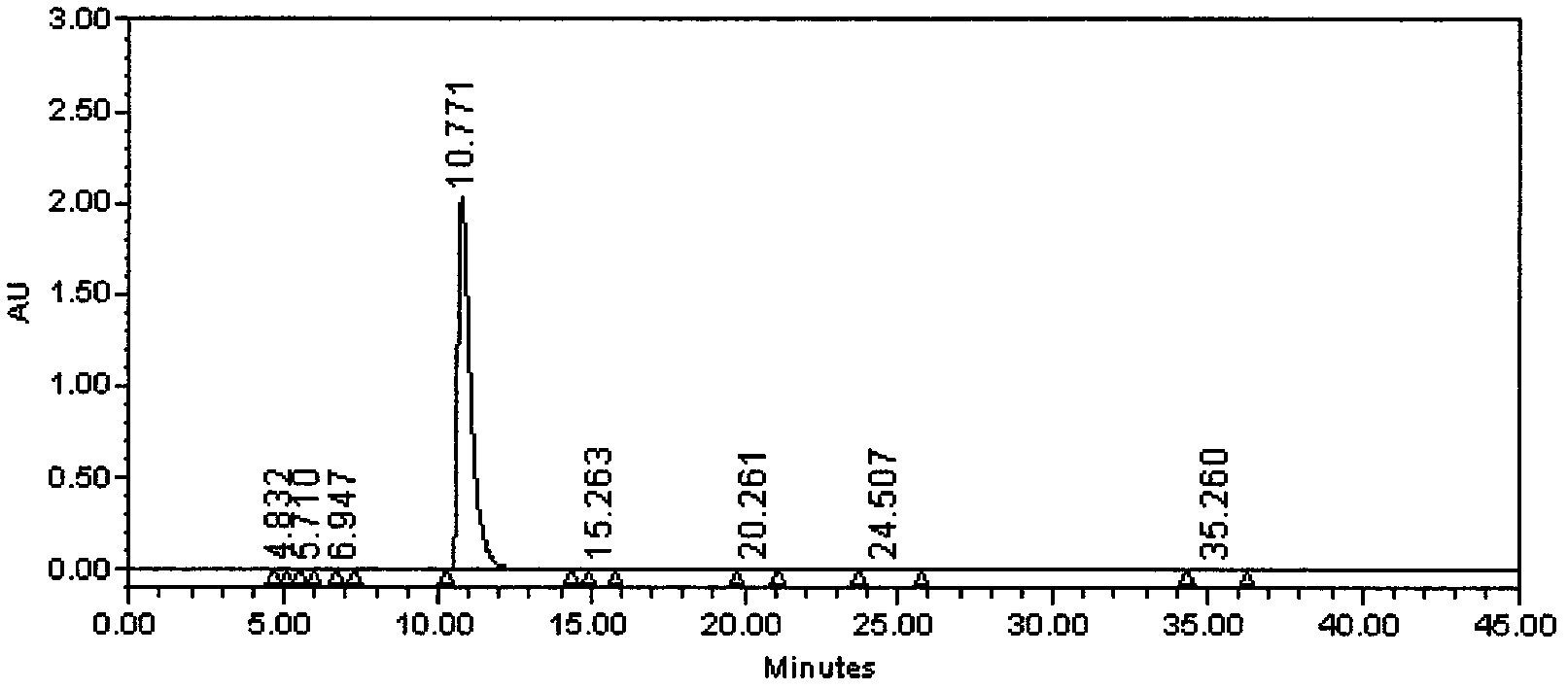
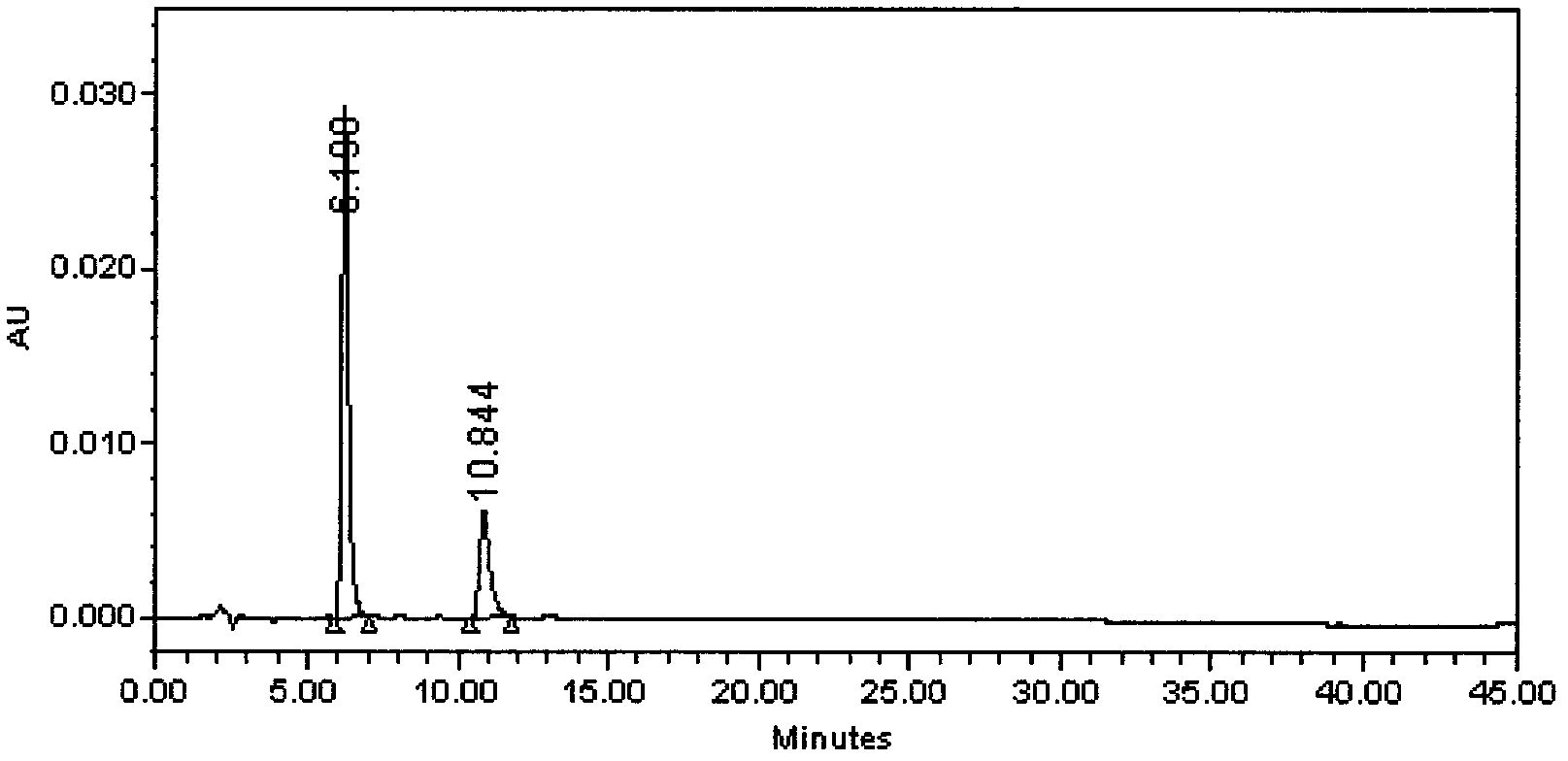
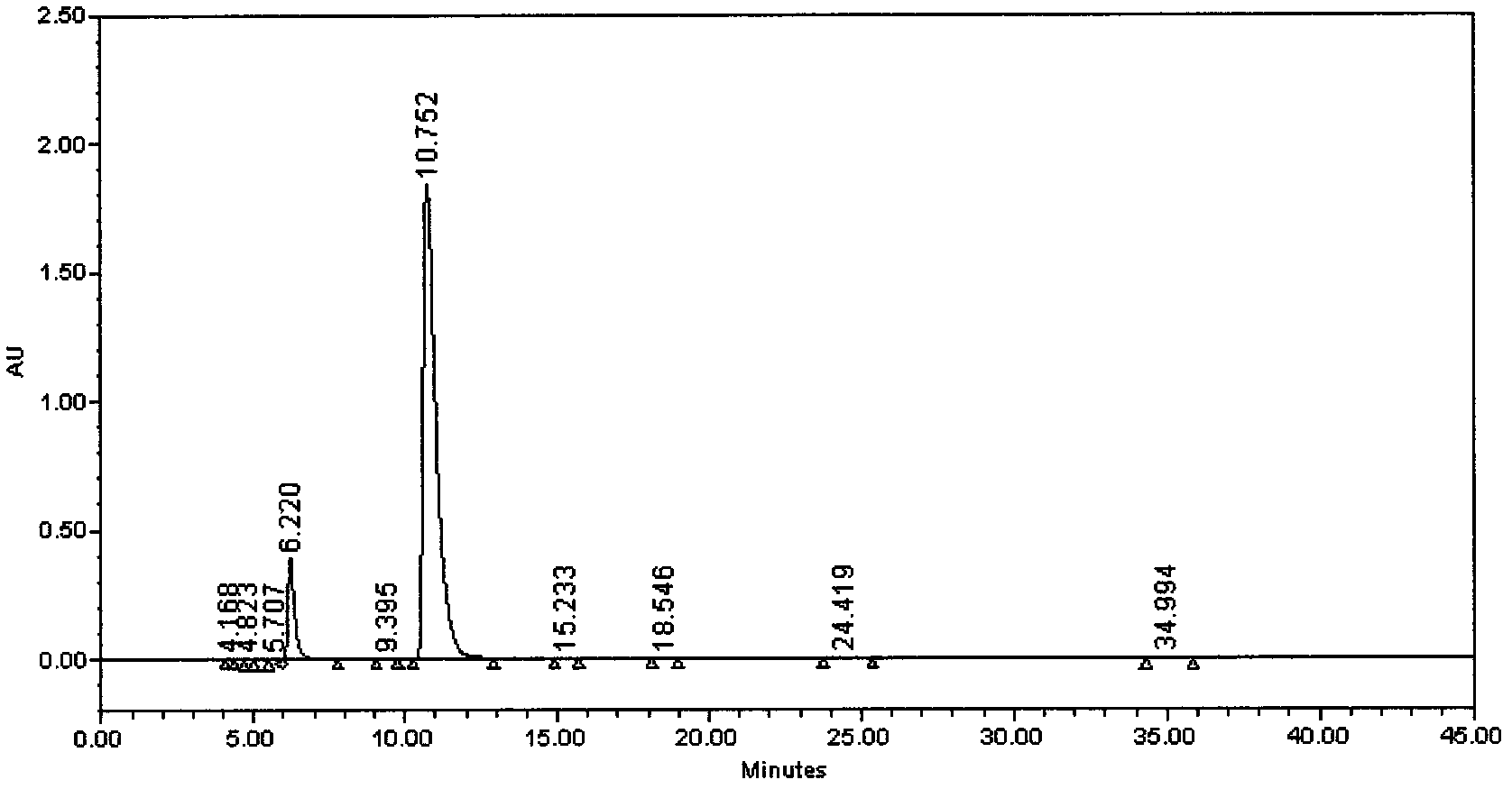
[0034] Example 2, (Z)-4-(2,4-fluorophenyl)-piperidinyl ketone oxime 5.0g, absolute ethanol 50ml, add 4-(3-chloropropyloxy)-3- Methoxyacetophenone 4.58g, Potassium iodide 2.16g, 2% KOH 176.5g, TLC detection reaction, heating up to 60°C for 14 hours, heating up to 70°C, reacting for 1.5 hours, cooling down to room temperature, precipitated solid, filtered, 10ml Wash with water to obtain crude iloperidone. The crude product was dissolved by heating with 120ml of ethanol, 1g of activated carbon was added, stirred, filtered hot, the filtrate was cooled to room temperature, and filtered to obtain 7.35g of the product with a yield of 91.2% and a purity of 99.4%.
Embodiment 3
[0035] Example 3, (Z)-4-(2,4-difluorophenyl)-piperidinyl ketone oxime 21.0g, acetonitrile 210ml, was added to a 500ml four-necked flask, and 20.1g 4-(3-chloro Propyloxy)-3-methoxyacetophenone, potassium iodide 1.5g, potassium hydroxide 7.3g was added, the temperature was raised to reflux for 26 hours, and the reaction was detected by TLC. After the reaction is completed, cool down to room temperature, pour the reaction solution into 700ml of water, and cool down to about 20°C±2°C. Stir for 4 hours, filter with suction, and wash the filter cake with 35ml of water. The product was dried at 50°C±2°C for 24 hours under a vacuum of 0.090Mpa to obtain 33.5g of a white solid with a yield of 94.5% and a purity of 99.4%. .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com