New preparation method of iloperidone
A technology of iloperidone and ethyl ketone, which is applied in the field of anti-schizophrenia drugs, can solve the problems of low yield, long cycle, and increased production cost, and achieve the effects of high yield, low cost, and shortened reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
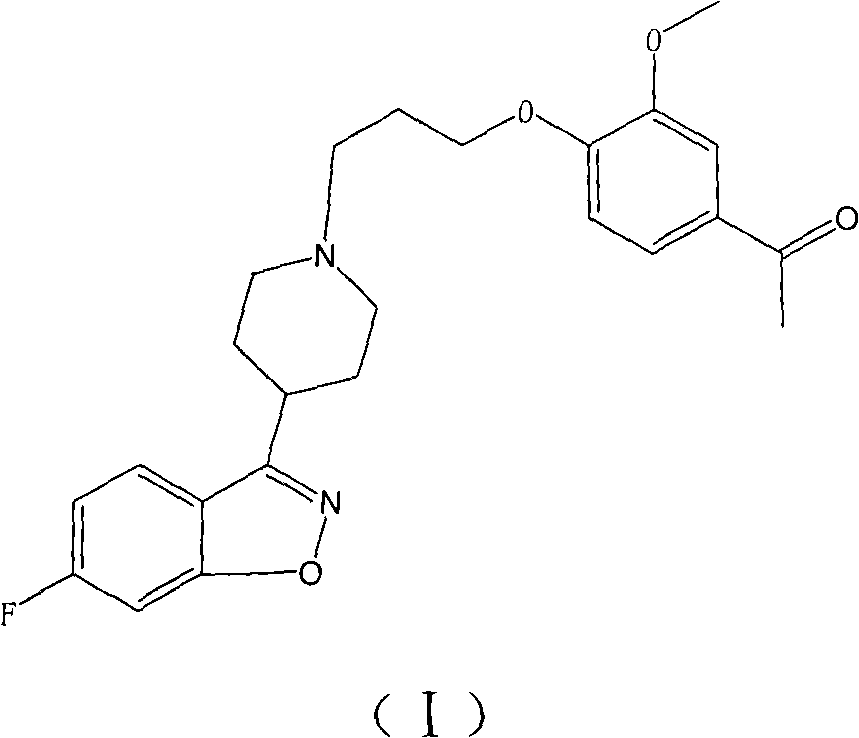
[0022] 1-(4-(3-(4-(6-fluoro-1,2-benzisoxazol-3-yl)piperidinyl)propoxy)-3-methoxyphenyl)ethylketone ( iloperidone) preparation
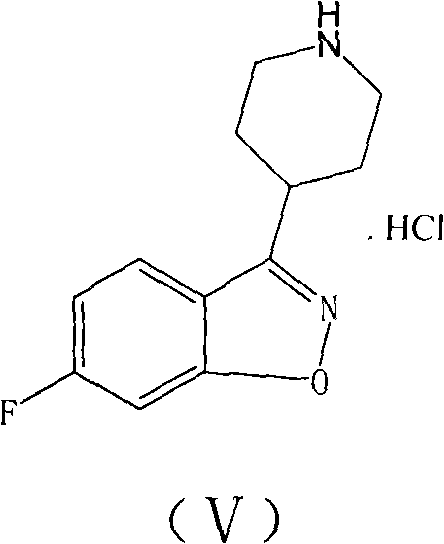
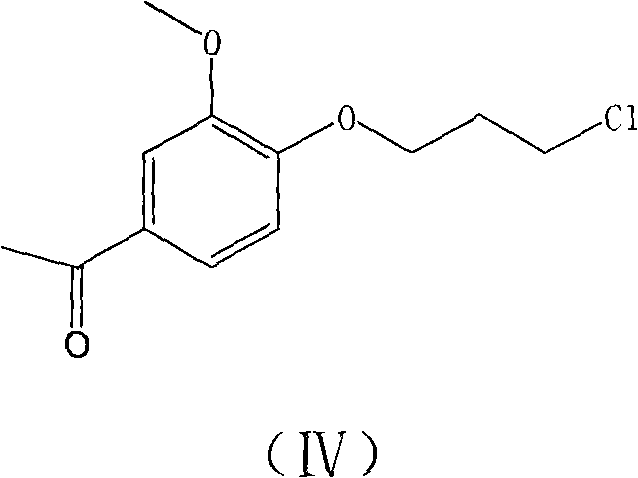
[0023] 10g reactant 6-fluoro-3-(4-piperidinyl)-1,2-benzisoxazole hydrochloride and 10.4g reactant 1-[4-(3-chloropropoxy)-3 -Put methoxyphenyl] ethyl ketone into a 250ml reaction bottle, add 17.9g potassium carbonate and 120ml water solution, heat the reaction mixture to 80-90°C and stir for 1.5 hours, then naturally cool to room temperature while stirring, filter , the filter cake was washed twice with water, then washed twice with methanol, and dried in vacuum at 50° C. to obtain 15.1 g of crude iloperidone, with a yield of 91.0%. The crude product was decolorized by activated carbon and recrystallized from toluene to obtain iloperidone, with a purity of 99.5% (as determined by HPLC), and a melting point of 118-120°C.
[0024] Implementation column 2
[0025] 1-(4-(3-(4-(6-fluoro-1,2-benzisoxazol-3-yl)piperidinyl)propoxy)-3-methoxyphenyl)ethylketo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com