Iloperidone sustained release microsphere and preparation method thereof
A technology for slow-release microspheres and iloperidone, applied in the field of medicine, can solve the problems of uneven release, poor particle uniformity, irregular shape of the microspheres, etc., and achieve the effects of good uniformity, stable release and regular shape.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
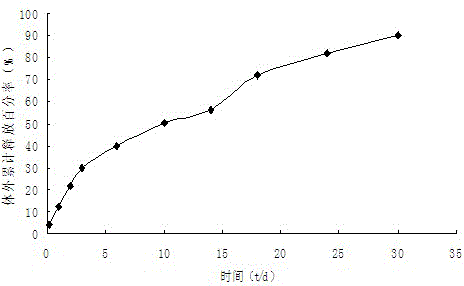
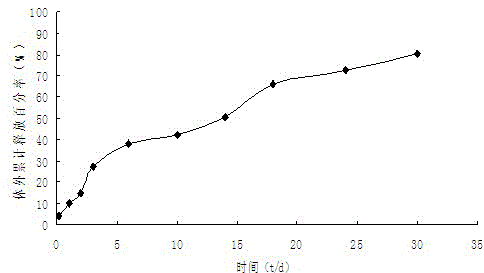
[0027] Weigh 200.21mg of PLGA (50 / 50, molecular weight 32000), 20.16mg of iloperidone, add 5mL of dichloromethane to stir and dissolve the clear solution to form an oil phase (O), quickly drop the oil phase into 75mL containing 1% In the PVA water phase (W), it was emulsified at 10,000 rpm for 90 s at high speed to form an O / W emulsion, and then the water phase was diluted with purified water by one time, and the obtained O / W emulsion was placed on a magnetic stirrer for Stir at 500rpm for 6 hours to volatilize and remove the organic solvent to solidify the microspheres, collect the microspheres by centrifugation, wash with purified water for 3 times, and then vacuum freeze-dry to obtain iloperidone microspheres. The drug loading was 7.61%, and the encapsulation efficiency was 83.18%.
Embodiment 2-7
[0029] Its prescription and process conditions are shown in Table 1.
[0030] The prescription and processing condition of table 1 embodiment 2~7
[0031]
[0032] The microsphere preparation step of embodiment 2~7 is the same as embodiment 1
Embodiment 8-14
[0034]Its prescription and technological conditions are shown in Table 2.
[0035]
[0036] The prescription and processing condition of table 2 embodiment 8~14
[0037]
[0038] The microsphere preparation step of embodiment 8~14 is the same as embodiment 1
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com