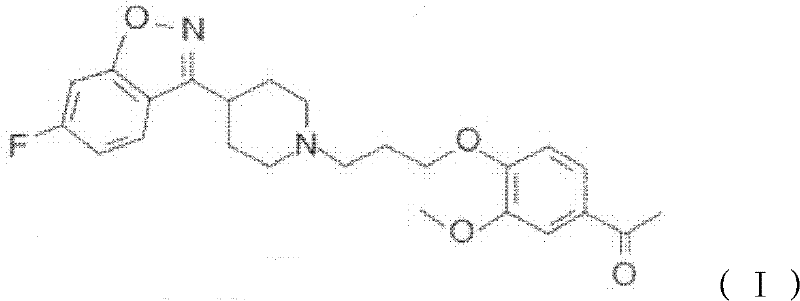
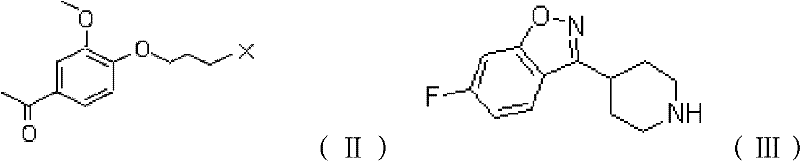Preparation method of antipsychotic drug iloperidone
A technology of iloperidone and ethyl ketone, which is applied in the field of anti-schizophrenia drugs, can solve the problems of low yield, high cost, and increased treatment of activated carbon waste residue, and achieve the effects of high yield, low cost, and shortened reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] React 4-hydroxy-3-methoxyacetophenone with 1-bromo-3-chloropropane in the solvent acetone and in the presence of potassium carbonate to obtain a crude product, which is purified with isopropyl ether to obtain 1-[4-(3 -chloropropoxy)-3-methoxyphenyl]ethanone.
[0033] With 26.670g (0.110mol) reactant 1-[4-(3-chloropropoxy)-3-methoxyphenyl] ethyl ketone and 25.650g (0.100mol) reactant 6-fluoro-3-(4- Piperidinyl)-1,2-benzisoxazole hydrochloride was placed in a 1000ml reaction flask, and 41.400g (0.300mol) of anhydrous potassium carbonate, 2.490g (0.015mol) of potassium iodide and 0.05% T 320.200g of the prepared solution in an aqueous solution at a temperature of 80°C, stirred and mixed, heated to 70-85°C, and reacted with stirring for 3-5 hours. Naturally cool to room temperature, then cool down to 15±5°C and keep for 1 hour. Suction filtration, wash the filter cake twice with 150ml of water, then wash with 50ml of cold anhydrous methanol, suction filtration to dryness,...
Embodiment 2
[0035]With 26.670g (0.110mol) reactant 1-[4-(3-chloropropoxy)-3-methoxyphenyl] ethyl ketone and 25.650g (0.100mol) reactant 6-fluoro-3-(4- Piperidinyl)-1,2-benzisoxazole hydrochloride was placed in a 1000ml reaction flask, and 41.400g (0.300mol) of anhydrous potassium carbonate, 2.490g (0.015mol) of potassium iodide and 0.05% T 320.200g of the prepared solution in an aqueous solution at a temperature of 60°C, stirred and mixed, heated to 70-85°C, and reacted with stirring for 3-5 hours. Naturally cool to room temperature, then cool down to 15±5°C and keep for 1 hour. Suction filtration, wash the filter cake twice with 150ml of water, and then with 50ml of cold methanol, suction filtration to dryness, and vacuum drying at 50°C to obtain 40.00g of crude iloperidone with a yield of 93.79%. The crude product was recrystallized from methanol to obtain 37.01 g of iloperidone, with a purity of over 99.5% (HPLC normalization method), and a recrystallization yield of 92.52%.
Embodiment 3
[0037] With 26.670g (0.110mol) reactant 1-[4-(3-chloropropoxy)-3-methoxyphenyl] ethyl ketone and 25.650g (0.100mol) reactant 6-fluoro-3-(4- Piperidinyl)-1,2-benzisoxazole hydrochloride was placed in a 1000ml reaction flask, and 41.400g (0.300mol) of anhydrous potassium carbonate, 2.490g (0.015mol) of potassium iodide and 0.05% T 320.200g of the prepared solution in an aqueous solution at a temperature of 65°C, stirred and mixed, heated to 70-85°C, and reacted with stirring for 3-5 hours. Naturally cool to room temperature, then cool down to 15±5°C and keep for 1 hour. Suction filtration, wash the filter cake twice with 150ml of water, then wash with 50ml of cold methanol, suction filtration to dryness, and vacuum drying at 50°C to obtain 39.40g of crude iloperidone with a yield of 92.4%. The crude product was recrystallized from methanol to obtain 36.25 g of iloperidone, with a purity of over 99.5% (HPLC normalization method), and a recrystallization yield of 92.00%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com