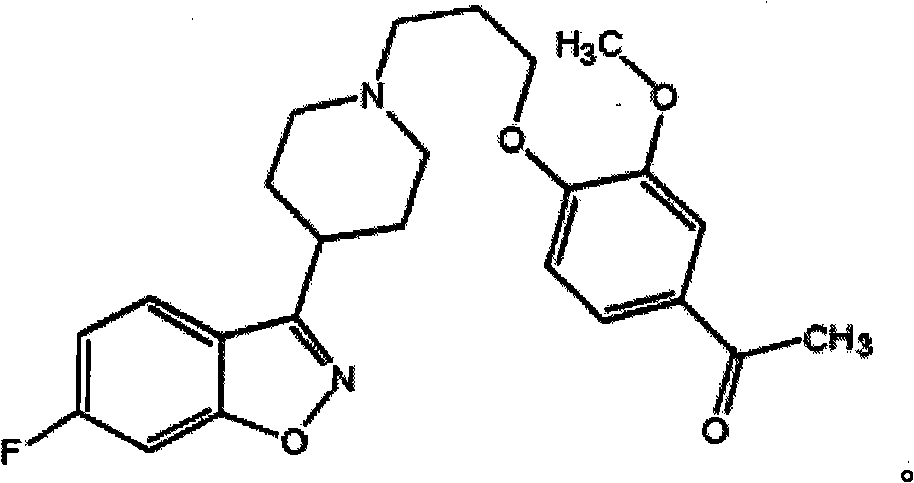
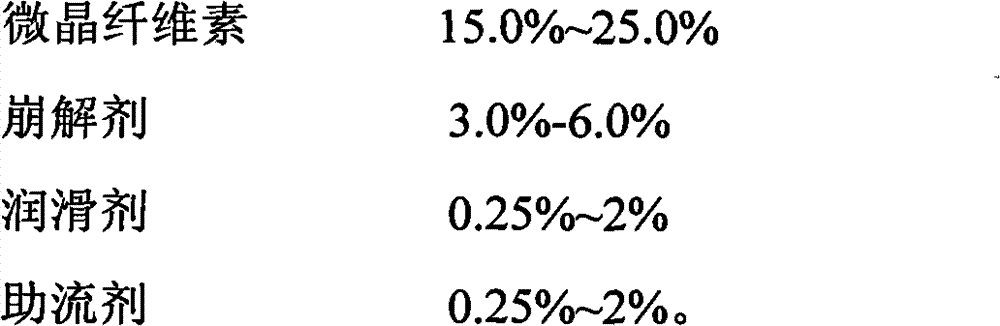Oral tablet containing iloperidone, and its preparation method
A technology for oral tablets of iloperidone and iloperidone, which is applied in the field of pharmaceutical preparations and can solve problems such as complex processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
Appropriate amount
[0047] Preparation process: Weigh a certain amount of purified water as a wetting agent; (V,0.9) =9μm iloperidone, lactose, microcrystalline cellulose, hypromellose, and internally added crospovidone were placed in a wet granulator and mixed uniformly to obtain mixture A; an appropriate amount of purified water was added to mixture A to prepare granules to obtain wet granules; the wet granules were sized and dried to obtain dry granules; the dry granules were evenly mixed with crospovidone, silicon dioxide, and magnesium stearate and then pressed into tablets; tablet weight 370mg, compressed into tablets Hardness 3-8kp.
[0048] Measure the iloperidone tablets prepared in Comparative Example 1 of the present invention, Comparative Example 2, Comparative Example 3, Comparative Example 4, Example 1 according to the disintegration time limit inspection method (two appendices of Chinese Pharmacopoeia version in 2010) The disintegration time in water, t...
Embodiment 2
[0057] components
[0058] Preparation process: Weigh a certain amount of purified water as a wetting agent; (V,0.9) = 4.5μm Iloperidone, lactose, microcrystalline cellulose, hypromellose, and internally added crospovidone are placed in a wet granulator and mixed uniformly to obtain mixture A; add an appropriate amount of purified water to mixture A Granulate to obtain wet granules; granulate and dry the wet granules to obtain dry granules; mix the dry granules with external crospovidone, silicon dioxide, and magnesium stearate evenly and then press into tablets; tablet weight 90mg, press Tablet hardness 1-4kp.
Embodiment 3
[0060] components
[0061] Crospovidone (additional)
[0062] Preparation process: Weigh a certain amount of purified water as a wetting agent; (V,0.9) =9μm iloperidone, lactose, microcrystalline cellulose, hypromellose, and internally added crospovidone were placed in a wet granulator and mixed uniformly to obtain mixture A; an appropriate amount of purified water was added to mixture A to prepare granules to obtain wet granules; the wet granules were sized and dried to obtain dry granules; the dry granules were evenly mixed with crospovidone, silicon dioxide, and magnesium stearate and then pressed into tablets; tablet weight 140mg, compressed into tablets Hardness 2-5kp.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com