Aripiprazole orally dissolving film and preparation method thereof
An aripiprazole and mouth-dissolving film technology is applied in the field of drug film preparations and their preparation, which can solve the problems of physical strength failing to meet the requirements for use, physical properties failing to meet the requirements, being easy to break, broken and the like, and to solve labor protection problems. and environmental pollution, excellent bioavailability, good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
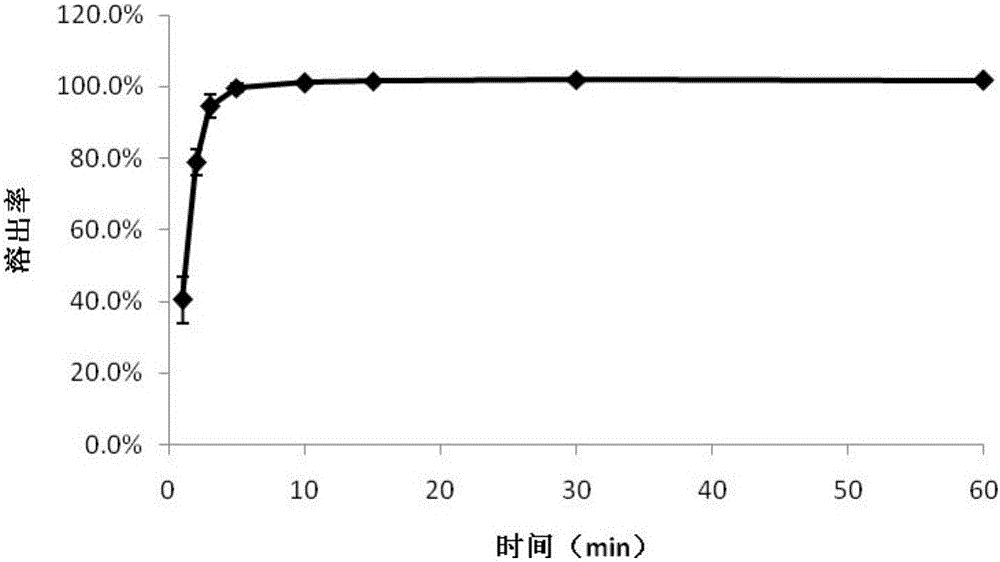
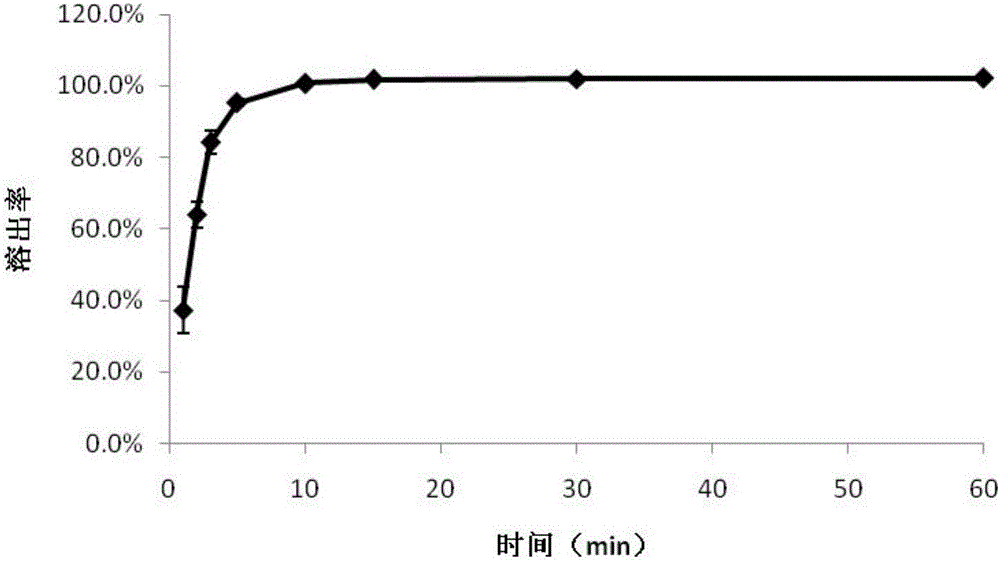
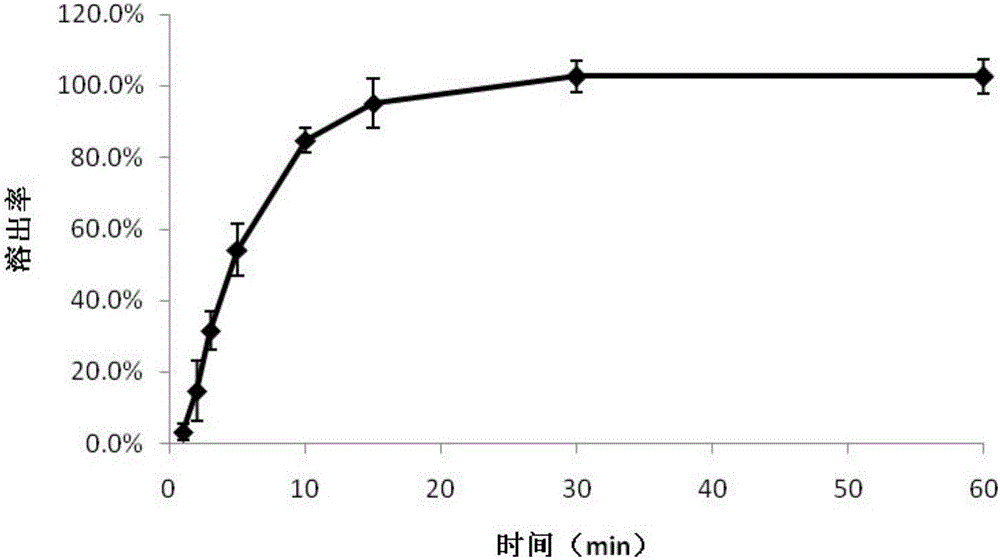
Image
Examples
Embodiment 1
[0047] prescription:
[0048]
[0049] Preparation:
[0050] Take hydroxypropyl-β-cyclodextrin and add 30ml of 0.1M hydrochloric acid solution, stir to dissolve, then add aripiprazole, stir at 60°C for 1 hour, then add 300ml of purified water, then add titanium dioxide, HPMC and HPC, and stir Uniformly form a high-viscosity solution, pass through an 80-mesh sieve, remove insoluble matter, and vacuum defoam. Then spread the membrane solution on a stainless steel belt and dry at 80°C for 15 minutes. According to the content measurement results, cut into a certain size, peel off from the stainless steel belt, and seal the package.
[0051] The obtained drug film preparation has a thickness of 0.065 mm, and each tablet contains 5 mg or 10 mg of aripiprazole. Oral once a day, the initial dose is 5mg / day in the first week, and increased to 10mg / day in the second week. After 2 weeks of medication, the dose can be increased according to the individual's curative effect and toleran...
Embodiment 2
[0058] prescription:
[0059]
[0060] Preparation:
[0061] Take glucosyl-β-cyclodextrin and add 24ml of 0.1M hydrochloric acid solution, stir to dissolve, then add aripiprazole, stir at 60°C for 1 hour, then add 300ml of purified water, then add the rest of the excipients, stir evenly to form a high viscosity The solution is passed through an 80-mesh sieve to remove insoluble matter, and vacuum defoaming. Then spread the membrane solution on a stainless steel belt and dry at 90°C for 15 minutes. According to the content measurement results, cut into a certain size, peel off from the stainless steel belt, and seal the package.
[0062] The obtained drug film preparation has a thickness of 0.065 mm, and each tablet contains 5 mg or 10 mg of aripiprazole. Oral once a day, the initial dose is 5mg / day in the first week, and increased to 10mg / day in the second week. After 2 weeks of medication, the dose can be increased according to the individual's curative effect and toler...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com