Pharmaceutical composition
A technology of calcium hydrogen phosphate dihydrate and eszopiclone, which is applied in the field of medicine, can solve the problems of large differences in the dissolution rate of eszopiclone tablets, and achieve the effect of improving the operating environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
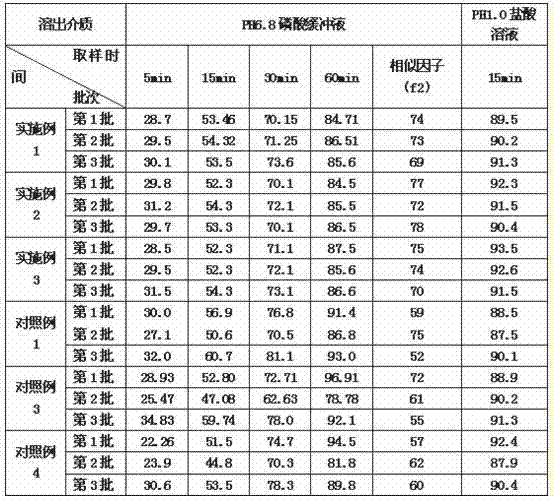
Embodiment 1
[0017] Embodiment 1, accurately weighed 3g of eszopiclone (using a jet mill to micronize it, control D 90 =30um), microcrystalline cellulose 113g, calcium hydrogen phosphate dihydrate 180g, croscarmellose sodium 3g, magnesium stearate 1.5g, mix well, and directly make 1000 tablets.
Embodiment 2
[0018] Embodiment 2, accurately weighed eszopiclone 3g (D 90 =30um), microcrystalline cellulose 60g, calcium hydrogen phosphate dihydrate 232.5g, croscarmellose sodium 3g, magnesium stearate 1.5g, mix well, and directly make 1000 tablets.
Embodiment 3
[0019] Embodiment 3, accurately weighed eszopiclone 3g (D 90 =30um), microcrystalline cellulose 292.5g, croscarmellose sodium 3g, magnesium stearate 1.5g, mix well, and directly make 1000 tablets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com