Nefopam hydrochloride tablet and preparation method thereof
A Nefopam Hydrochloride Tablet and Nefopam Hydrochloride Technology, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problem of affecting the efficacy of medicines, and Nefopam Hydrochloride Tablets. Coating treatment, poor quality control of the processing process, etc., to achieve the effect of consistency guarantee
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1, a kind of Nefopam hydrochloride tablet, is made of the raw material of following parts by weight:
[0028] 30 parts of calcium hydrogen phosphate dihydrate, 25 parts of microcrystalline cellulose, 1 part of hypromellose, 1 part of anhydrous silica gel, 1 part of magnesium stearate, 10 parts of Nefopam hydrochloride, 2 parts of white Opadry 20A58806 .
[0029] Preferably, include the following steps:
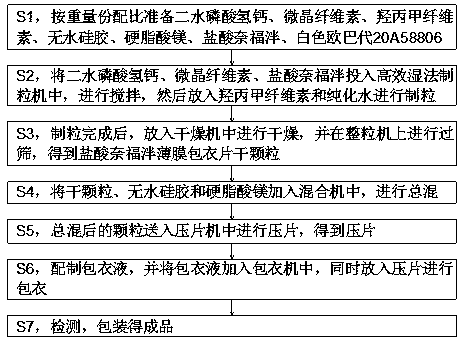
[0030] S1, preparing calcium hydrogen phosphate dihydrate, microcrystalline cellulose, hypromellose, anhydrous silica gel, magnesium stearate, Nefopam hydrochloride, and white Opadry 20A58806 according to the proportion by weight;
[0031] S2, putting calcium hydrogen phosphate dihydrate, microcrystalline cellulose, and Nefopam hydrochloride into a high-efficiency wet granulator, stirring, and then adding hypromellose and purified water to granulate;
[0032] S3, after the granulation is completed, put it into a drier for drying, and sieve it on a granulato...
Embodiment 2
[0042] Embodiment 2, a kind of Nefopam hydrochloride tablet, is made of the raw material of following parts by weight:
[0043] 40 parts of calcium hydrogen phosphate dihydrate, 30 parts of microcrystalline cellulose, 3 parts of hypromellose, 5 parts of anhydrous silica gel, 3 parts of magnesium stearate, 30 parts of Nefopam hydrochloride, 15 parts of white Opadry 20A58806 .
[0044] Include the following steps:
[0045] S1, preparing calcium hydrogen phosphate dihydrate, microcrystalline cellulose, hypromellose, anhydrous silica gel, magnesium stearate, Nefopam hydrochloride, and white Opadry 20A58806 according to the proportion by weight;
[0046] S2, putting calcium hydrogen phosphate dihydrate, microcrystalline cellulose, and Nefopam hydrochloride into a high-efficiency wet granulator, stirring, and then adding hypromellose and purified water to granulate;
[0047] S3, after the granulation is completed, put it into a drier for drying, and sieve it on a granulator to obt...
Embodiment 3
[0057] Embodiment 3, a kind of Nefopam hydrochloride tablet, is made of the raw material of following parts by weight:
[0058] 50 parts of calcium hydrogen phosphate dihydrate, 50 parts of microcrystalline cellulose, 5 parts of hypromellose, 6 parts of anhydrous silica gel, 3 parts of magnesium stearate, 30 parts of Nefopam hydrochloride, 15 parts of white Opadry 20A58806 .
[0059] Include the following steps:
[0060] S1, preparing calcium hydrogen phosphate dihydrate, microcrystalline cellulose, hypromellose, anhydrous silica gel, magnesium stearate, Nefopam hydrochloride, and white Opadry 20A58806 according to the proportion by weight;
[0061] S2, putting calcium hydrogen phosphate dihydrate, microcrystalline cellulose, and Nefopam hydrochloride into a high-efficiency wet granulator, stirring, and then adding hypromellose and purified water to granulate;
[0062] S3, after the granulation is completed, put it into a drier for drying, and sieve it on a granulator to obt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com