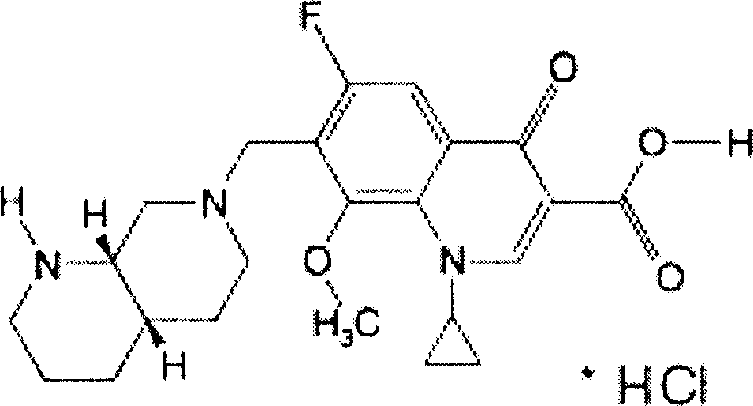
Moxifloxacin capsule and its preparation method
A technology for moxifloxacin and capsules is applied to a capsule dosage form of moxifloxacin or its salt and/or its hydrate and its preparation field, and achieves the effects of high dissolution rate and stable dissolution performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Ingredient: Moxifloxacin Hydrochloride 218.4mg
[0035] Microcrystalline Cellulose 40.0mg
[0036] Sodium starch glycolate 68.0mg
[0037] Magnesium Stearate 3.6mg
[0038]
[0039] Total 330.0mg
[0040] Preparation method: Take the raw materials and auxiliary materials in the above components, mix them well, and fill them into hydroxypropyl methylcellulose capsule shells.
[0041] The dissolution rate of the obtained capsules was measured. And after the obtained capsules were placed at room temperature for two years, the dissolution rate was measured by the same method, and the results are shown in Table 2 below.
Embodiment 2
[0043] Ingredient: Moxifloxacin Hydrochloride 436.8mg
[0044] Microcrystalline Cellulose 55.0mg
[0045] Carboxymethyl Starch Sodium 100.0mg
[0046] Magnesium Stearate 8.2mg
[0047]
[0048] Total 600.0mg
[0049] Preparation method: Take the raw materials and auxiliary materials in the above components, mix them well, make granules by dry method, and fill them into hydroxypropyl methylcellulose capsule shells.
[0050] The dissolution rate of the obtained capsules was measured. And after the obtained capsules were placed at room temperature for two years, the dissolution rate was measured by the same method, and the results are shown in Table 2 below.
Embodiment 3
[0052] Ingredient: Moxifloxacin Hydrochloride 218.4mg
[0053] Microcrystalline Cellulose 30.0mg
[0054] Sodium starch glycolate 68.0mg
[0055] Croscarmellose Sodium 10.0mg
[0056] Magnesium Stearate 3.6mg
[0057]
[0058] Total 330.0mg
[0059] Preparation method: Take the raw materials and auxiliary materials in the above components, mix them well, and fill them into hydroxypropyl methylcellulose capsule shells.
[0060] The dissolution rate of the obtained capsules was measured. And after the obtained capsules were placed at room temperature for two years, the dissolution rate was measured by the same method, and the results are shown in Table 2 below.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com