Dapagliflozin microencapsulated sustained-release tablet and preparation method thereof
A technology for sustained-release tablets and microcapsules, applied in the field of dapagliflozin microencapsulated sustained-release tablets and their preparation, can solve the problems of short action time, failure to achieve long-term blood sugar control, fast absorption, etc. Long-acting release, prolonging the intestinal action time, reducing the effect of toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
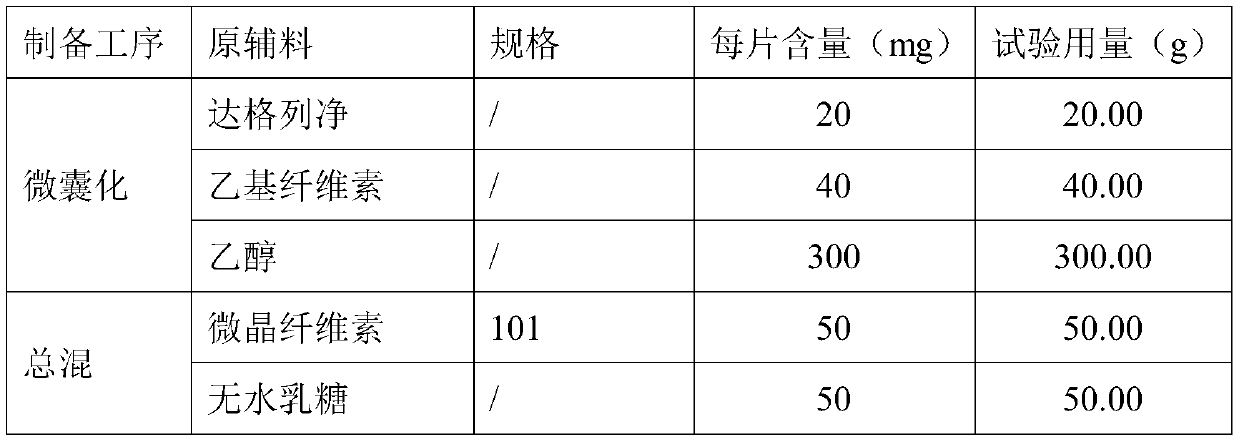
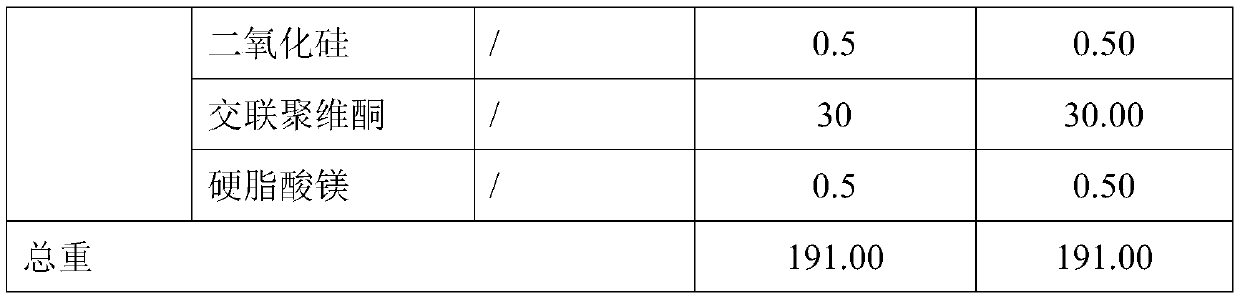
[0031]
[0032]
[0033] Its preparation method is
[0034] (1) Microencapsulation:
[0035] Mix the formulated amount of dapagliflozin and ethyl cellulose ethanol solution evenly, and spray-dry the above suspension. Working conditions: inlet temperature: 130-160°C, outlet temperature: 70-90°C, feeding speed: 20mL / min to obtain dry particles with a particle size of 600 μm.
[0036] (2) mixing:
[0037] Add the prepared microcapsules, microcrystalline cellulose, anhydrous lactose, silicon dioxide, crospovidone, and magnesium stearate into a hopper mixer and mix evenly;
[0038] (3) Tablets:
[0039] The uniformly mixed intermediate product in (2) is selected from a circular shallow concave die and pressed into tablets, the weight of the tablet is controlled to be about 191 mg, and the hardness range is 6-12 kg, and finally a microencapsulated sustained-release tablet containing dapagliflozin is obtained.
[0040] It should be noted that, as an alternative implementat...
Embodiment 2
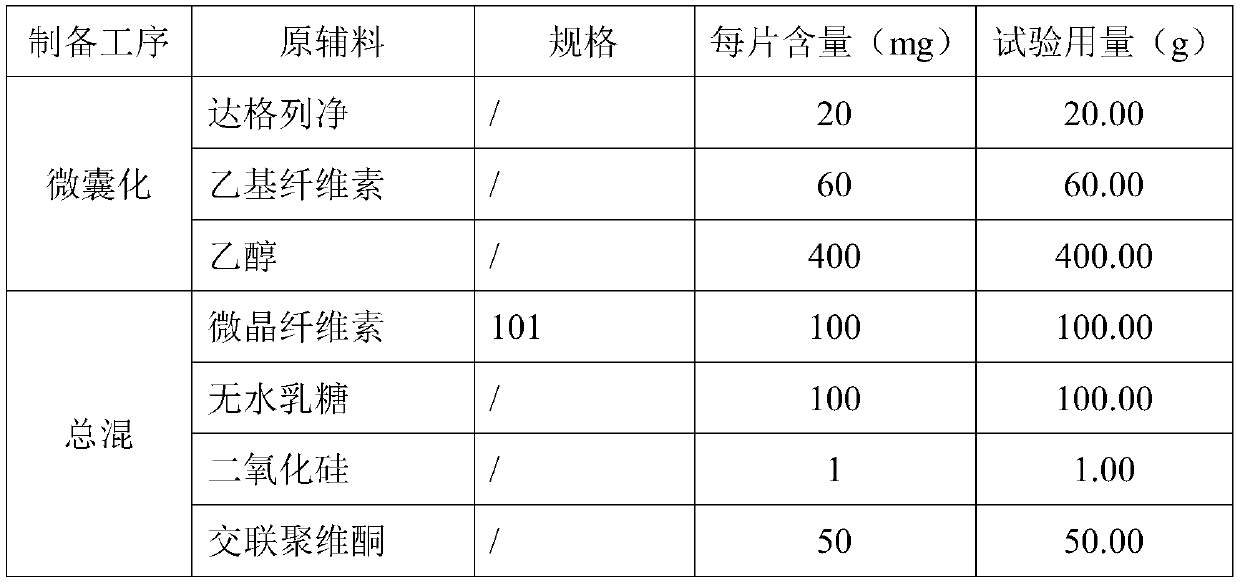
[0043]
[0044]
[0045] Its preparation method is
[0046] (2) Microencapsulation:
[0047] Mix the formula amount of dapagliflozin and ethyl cellulose ethanol solution evenly, and spray the above suspension to dry. Working conditions: inlet temperature: 130-160°C, outlet temperature: 70-90°C, feeding speed: 20ml / min to obtain dry particles with a particle size of 600 μm.
[0048] (2) mixing:
[0049] Add the prepared microcapsules, cornstarch, silicon dioxide, low-substituted cellulose, and stearic acid into a hopper mixer and mix evenly;
[0050] (3) Tablets:
[0051] The uniformly mixed intermediate product in (2) is selected from a circular shallow concave die and pressed into tablets, the weight of the tablet is controlled to be about 332 mg, and the hardness range is 6-12 kg, and finally a microencapsulated sustained-release tablet containing dapagliflozin is obtained.
Embodiment 3
[0053]
[0054] Its preparation method is
[0055] (3) Microencapsulation:
[0056] Mix the formula amount of dapagliflozin and ethyl cellulose ethanol solution evenly, and spray the above suspension to dry. Working conditions: inlet temperature: 130-160°C, outlet temperature: 70-90°C, feeding speed: 20ml / min to obtain dry particles with a particle size of 600 μm.
[0057] (2) mixing:
[0058] Add the prepared microcapsules, microcrystalline cellulose, anhydrous lactose, low-substituted cellulose, and stearic acid into a hopper mixer and mix evenly;
[0059] (3) Tablets:
[0060] The uniformly mixed intermediate product in (2) is selected from a circular shallow concave die and pressed into tablets. The weight of the tablet is controlled to be about 181mg, and the hardness range is 6-12kg. Finally, a microencapsulated sustained-release tablet containing dapagliflozin is obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com