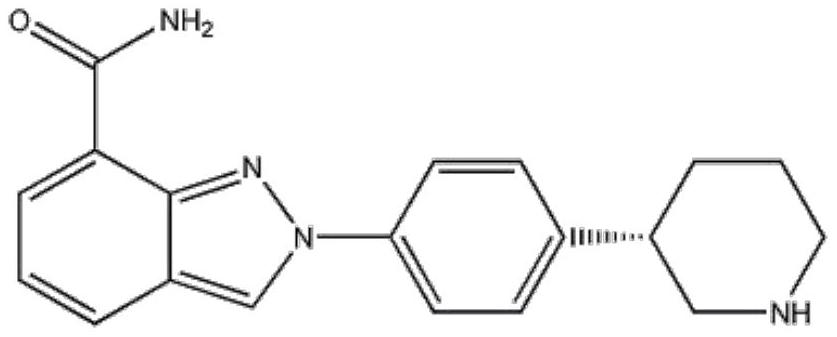
Method for preparing Niraparib microcapsule preparation
A preparation and capsule material technology, applied in the field of anticancer drug microcapsule preparations, niraparib microcapsule preparations and their preparation fields, can solve the problems of low drug loading, limited wide use, poor encapsulation rate and the like, and achieve dissolution The effect of stable, increasing drug loading and improving encapsulation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
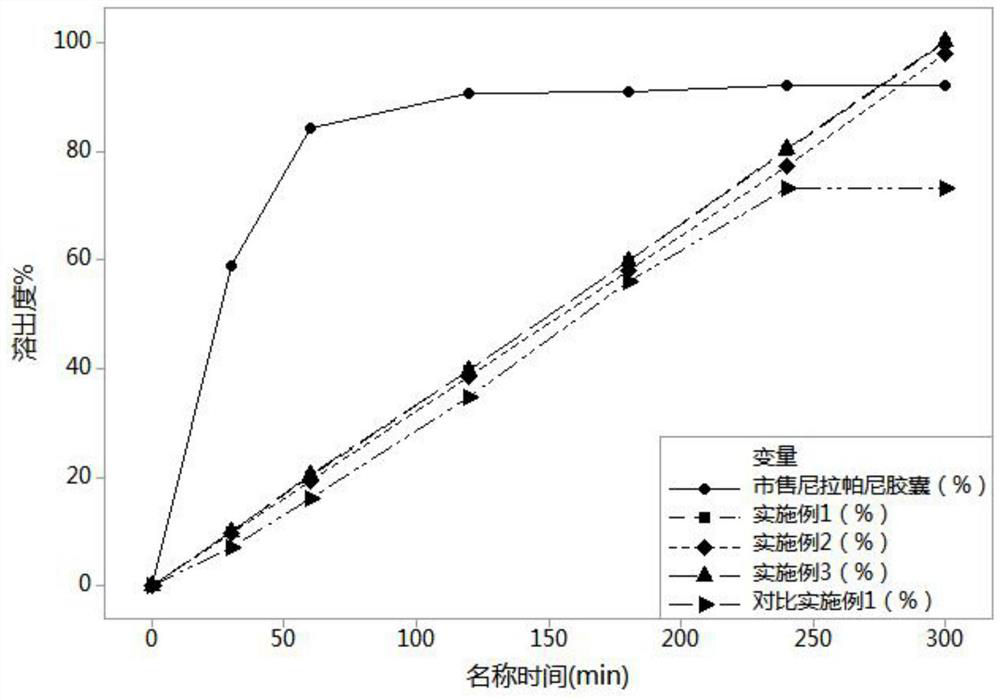
Image
Examples
Embodiment 1
[0037] Embodiment 1: the preparation of niraparib capsules of the present invention
[0038]
[0039] Preparation Process:
[0040] ① Mix and pulverize niraparib and boric acid to 80 meshes, place in a fluidized bed, pass hot air into it to suspend and fluidize it, and the temperature of the hot air is 65-75°C;
[0041] ②Add polyvinyl alcohol into purified water at 65-75°C while stirring, and prepare a polyvinyl alcohol solution with a mass percentage of 5-10%.
[0042] ③ Atomize the polyvinyl alcohol solution of the capsule material through the nozzle of the fluidized bed and continuously add it to the fluidized bed. The atomization pressure is 0.2-0.4Mpa; the conveying speed is 15-20r / min; Moisture content <1.5%, stop heating, cool and discharge, and obtain niraparib microcapsules;
[0043] 4. Mix the niraparib microcapsules with the prescribed amount of copovidone S-630 and dextrin evenly, and directly fill to obtain the niraparib microcapsule capsules of the present i...
Embodiment 2
[0044] Embodiment 2: the preparation of niraparib microcapsule capsule of the present invention
[0045]
[0046] Preparation Process:
[0047] ① Mix and pulverize niraparib and boric acid to 80 meshes, place in a fluidized bed, pass hot air into it to suspend and fluidize it, and the temperature of the hot air is 65-75°C;
[0048] ②Add polyvinyl alcohol into purified water at 65-75°C while stirring, and prepare a polyvinyl alcohol solution with a mass percentage of 5-10%.
[0049]③ Atomize the polyvinyl alcohol solution of the capsule material through the nozzle of the fluidized bed and continuously add it to the fluidized bed. The atomization pressure is 0.2-0.4Mpa; the conveying speed is 15-20r / min; Moisture content <1.5%, stop heating, cool and discharge, and obtain niraparib microcapsules;
[0050] 4. mix the niraparib microcapsules and the prescription amount of crospovidone and xylitol evenly, and directly fill to obtain the niraparib microcapsule capsules of the p...
Embodiment 3
[0051] Embodiment 3: the preparation of niraparib microcapsule capsule of the present invention
[0052]
[0053] Preparation Process:
[0054] ① Mix and pulverize niraparib and boric acid to 80 meshes, place in a fluidized bed, pass hot air into it to suspend and fluidize it, and the temperature of the hot air is 65-75°C;
[0055] ②Add polyvinyl alcohol into purified water at 65-75°C while stirring, and prepare a polyvinyl alcohol solution with a mass percentage of 5-10%.
[0056] ③ Atomize the polyvinyl alcohol solution of the capsule material through the nozzle of the fluidized bed and continuously add it to the fluidized bed. The atomization pressure is 0.2-0.4Mpa; the conveying speed is 15-20r / min; Moisture content <1.5%, stop heating, cool and discharge, and obtain niraparib microcapsules;
[0057] 4. Mix the niraparib microcapsules with the prescribed amount of microcrystalline cellulose and pregelatinized starch evenly, and directly fill to obtain the niraparib mi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com