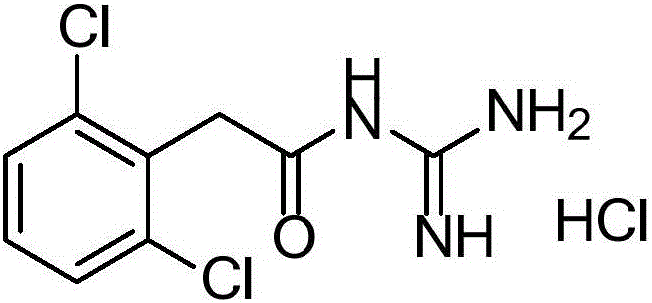
Guanfacine implant and preparing method thereof
An implant, guanfacine technology, applied in the field of guanfacine implant and preparation, can solve liver toxicity, leg cramps, sudden high blood pressure, high peak blood drug concentration of common dosage form release rate, plasma protein The problem of high binding rate can achieve the effect of small batch difference, good stability and smooth appearance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
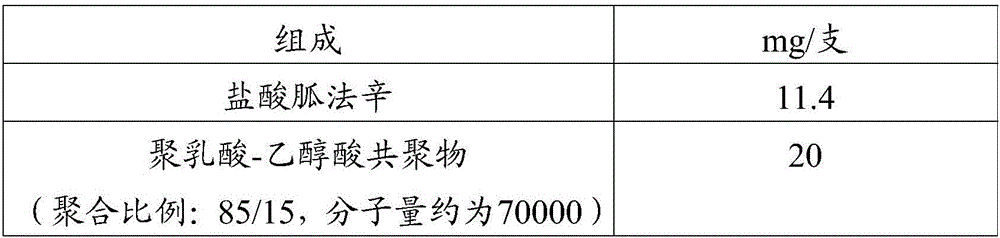
Embodiment 1
[0043] Prescription (specification: 10mg / bottle, calculated as free base):
[0044]
[0045] preparation:
[0046] Weighing the prescription amount of guanfacine hydrochloride and dissolving it in an appropriate amount of methanol; weighing the prescription amount of polylactic acid-glycolic acid and dissolving it in an appropriate amount of acetone; mixing the above two solutions and spray drying to obtain solid particles; The solid particles were added into a twin-screw hot-melt extruder, and the extrusion temperature was set at 75° C. to obtain a long cylindrical preparation; the prepared preparation was sterilized by radiation, and then packaged in a clean area.
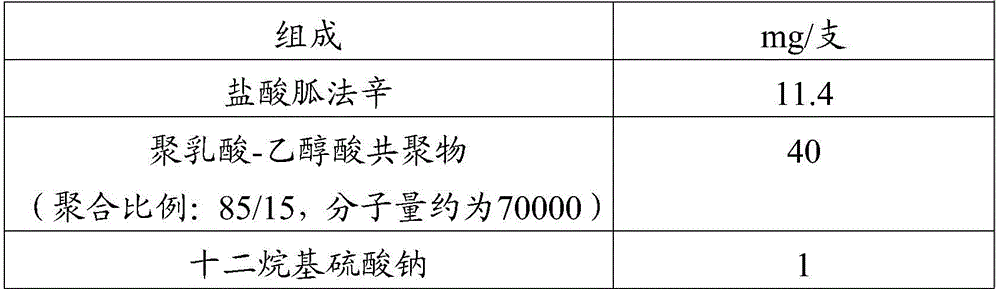
Embodiment 2
[0048] Prescription (specification: 10mg / bottle, calculated as free base):
[0049]
[0050] preparation:
[0051] Weigh the prescribed amount of guanfacine hydrochloride, polylactic acid-glycolic acid copolymer and sodium lauryl sulfate, and mix them uniformly in a mixer to obtain a mixed material; add the mixed material into a twin-screw hot-melt extruder, The extrusion temperature was set at 70° C. to prepare long cylindrical preparations; the prepared preparations were sterilized by radiation and then packaged in a clean area.
Embodiment 3
[0053] Prescription (specification: 10mg / bottle, calculated as free base):
[0054]
[0055] preparation:
[0056]Weigh the prescribed amount of guanfacine hydrochloride and polyethylene glycol-poly(lactic-co-glycolic acid) block copolymer, and mix them uniformly in a mixer to obtain a mixed material; put the mixed material into a twin-screw hot-melt extruder, extrude The outlet temperature was set at 80° C. to prepare long cylindrical preparations; the prepared preparations were sterilized by radiation, and then packaged in a clean area.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com