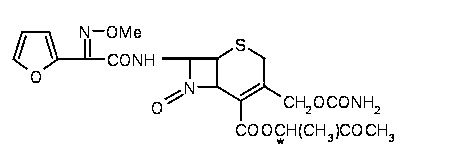
Preparation method of cefuroxime axetil
A technology of cefuroxime axetil and cefuroxime axetil, which is applied in the field of preparation of cefuroxime axetil, can solve the problems of long reaction time, harsh operating conditions, and many impurities in production, and achieve shortened production cycle, mild process conditions, and improved product quality. quality effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Add 150 mL of dimethylformamide to the reaction flask, add 15 g of cefuroxime acid under stirring, stir and dissolve until clear, cool the solution to 0°C; slowly add 15 mL of 1-bromoethyl acetate dropwise; when 1-bromoethyl After the diethyl acetate was added dropwise, 1.5g of copper chloride catalyst was added, and the temperature of the solution was raised to 15°C to continue the stirring reaction for 0.5 hours. The reaction process was monitored by HPLC, and the reaction residue of cefuroxime acid was controlled to be ≤1.0%;
[0041] After the reaction is complete, add 120ml of ethyl acetate under stirring and stir for 15 minutes. After completion, add 10% sodium chloride solution and stir for extraction for 20 minutes. Leave to stand and separate, remove the lower aqueous phase to obtain the upper organic phase and transfer it to the reaction bottle Add 90mL of 3% hydrochloric acid aqueous solution to the organic phase again, stir and wash for 20 minutes, let stand ...
Embodiment 2
[0044] Add 150 mL of dimethylformamide to the reaction flask, add 16.5 g of cefuroxime acid under stirring, stir and dissolve until clear, cool the solution to 2°C; slowly add 15 mL of 1-bromoethyl acetate; when 1-bromo After the ethyl acetate was added dropwise, 1.7 g of copper chloride catalyst was added, and the temperature of the solution was raised to 20° C. to continue stirring and reacting for 1.0 hour. The reaction process was monitored by HPLC, and the reaction residue of cefuroxime acid was controlled to be ≤1.0%;
[0045] After the reaction is complete, add 148.5ml of ethyl acetate under stirring and stir for 15 minutes. After completion, add 10% sodium chloride solution, stir and extract for 20 minutes, let stand for stratification, and remove the lower aqueous phase to obtain the upper organic phase and transfer it to the reaction bottle Add 99 mL of 3% hydrochloric acid aqueous solution to the organic phase again, stir and wash for 20 minutes, let stand to separat...
Embodiment 3
[0048] Add 150 mL of dimethylformamide to the reaction flask, add 18 g of cefuroxime acid under stirring, stir and dissolve until clear, cool the solution to 5°C; slowly add 21.6 mL of 1-bromoethyl acetate; when 1-bromoethyl acetate After the ethyl acetate was added dropwise, 2.3 g of copper chloride catalyst was added, and the temperature of the solution was raised to 25° C. to continue stirring and reacting for 1.0 hour. The reaction process was monitored by HPLC, and the reaction residue of cefuroxime acid was controlled to be ≤ 1.0%;
[0049] After the reaction is complete, add 180ml of ethyl acetate under stirring and stir for 15 minutes. After completion, add 10% sodium chloride solution and stir for extraction for 20 minutes. Let stand to separate layers, remove the lower phase water phase to obtain the upper phase organic phase and transfer it to the reaction bottle Add 135mL3% hydrochloric acid aqueous solution to the organic phase again, stir and wash for 20 minutes, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com