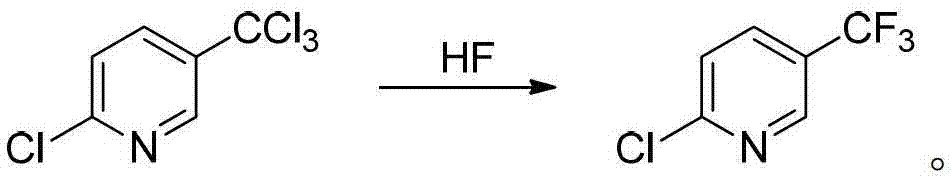
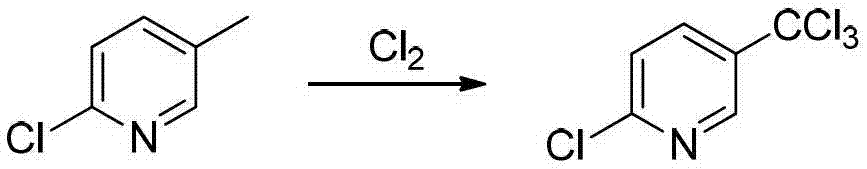
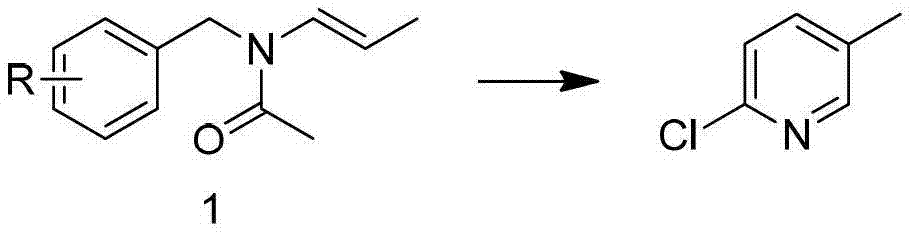
Preparation method of 2-chloro-5-tirfluoromethylpyridine
A technology of trifluoromethylpyridine and trichloromethylpyridine, applied in the field of preparation of 2-chloro-5-trifluoromethylpyridine, which can solve the problems of dangerous operation, many reaction by-products, and poor atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0127] (1) Step 1: N 2 After testing the airtightness of the autoclave, in a 500mL autoclave, add 39.2g of benzyl chloride and 80.6g of 32% NaOH aqueous solution in succession (mass percentage means that the quality of sodium hydroxide accounts for the total amount of the aqueous solution of sodium hydroxide mass percentage), lower the temperature to 5°C to 15°C, add 42.2g of ammonia water with a mass percentage of 25% (mass percentage refers to the percentage of the mass of ammonia gas in the total mass of the ammonia solution), and control the temperature at 5°C to 15°C, after the dropwise addition, the temperature was raised to 60°C (at this time, the pressure in the kettle was about 0.25MPa), and after keeping at this temperature for 7 hours, samples were taken, and the content of benzyl chloride was detected by HPLC to be less than 0.3%. ×2 toluene extraction, combined organic phase, using 30% hydrochloric acid by mass percentage (mass percentage refers to the mass percen...
Embodiment 2
[0133] (1) Step 1: N 2 After testing the tightness of the autoclave, in a 500mL autoclave, successively add 49.8g of o-chlorobenzyl chloride, 70.6g of toluene and 90.3g of KOH solution with a mass percentage of 40% (mass percentage refers to the mass of potassium hydroxide percentage of the total mass of potassium hydroxide aqueous solution), lower the temperature to 5°C to 15°C, and add 42.2g of ammonia water with a mass percentage of 25% (mass percentage refers to the percentage of the mass of ammonia gas in the total mass of ammonia solution), when adding Control the temperature at 5°C to 15°C, raise the temperature to 45°C after the dropwise addition (at this time, the pressure in the kettle is about 0.15MPa), keep the temperature at this temperature for 10 hours, take a sample, and determine that the content of o-chlorobenzyl chloride is less than 0.3% by HPLC. After the reaction is over, the reaction solution is extracted with 40g×2 toluene, the organic phases are combin...
Embodiment 3
[0139] (1) N 2 After testing the airtightness of the autoclave, in a 500mL autoclave, add 43.5g of m-methylbenzyl chloride and 80.6g of NaOH solution with a mass percentage of 32% (mass percentage means that the mass percentage of sodium hydroxide accounts for the percentage of sodium hydroxide percentage of the total mass of the aqueous solution), lower the temperature to 5°C to 15°C, add 42.2g of ammonia water with a mass percentage of 25% (mass percentage refers to the percentage of the mass of ammonia gas in the total mass of the ammonia solution), and control the temperature at 5 ℃~15℃, after the dropwise addition, the temperature is raised to 60℃ (at this time, the pressure in the kettle is about 0.25MPa), after keeping at this temperature for 9h, take a sample, and the HPLC detection of m-methylbenzyl chloride content is less than 0.3%, confirming that the reaction is over. The reaction solution was extracted with 60g×2 toluene, the organic phases were combined, and the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com