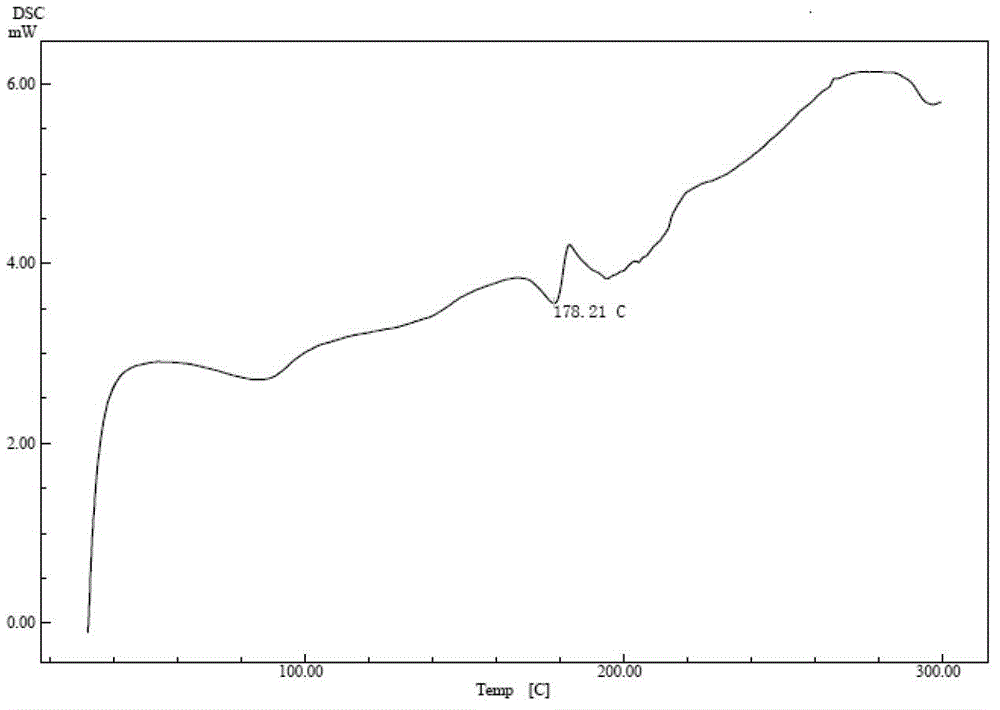
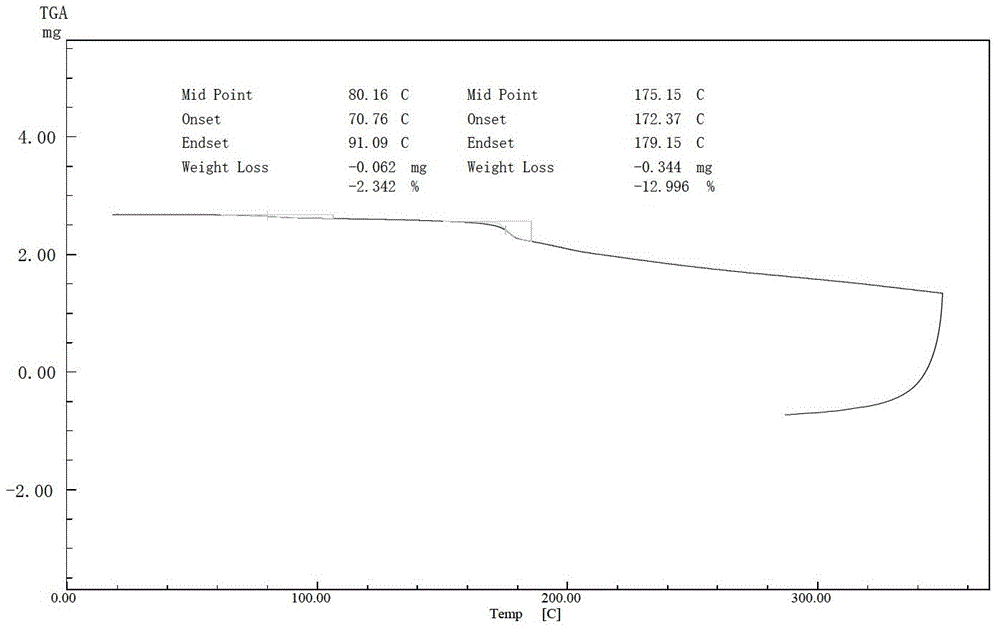
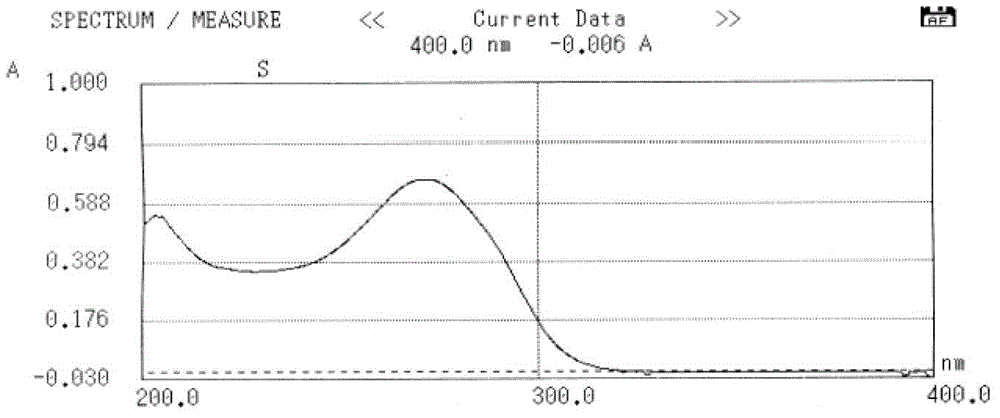
Cefuroxime lysine and preparation thereof
A technology for cefuroxime lysine and cefuroxime acid, which is applied in the preparation of cyanide reaction, the preparation of organic compounds, and medical preparations containing active ingredients, etc., can solve the problems of many impurities and low purity, and achieve high purity , the effect of strong stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Example 1 Preparation of 3-decarbamoyl-acetyl-cefuroxim sodium:
[0078]
[0079] (1) Preparation of 7-aminocephalosporanic acid crystals:
[0080] a-1. Weigh 7-aminocephalosporanic acid and add it to 30 times the weight of water to form a suspension, add 4mol / l ammonia water, stir until the 7-aminocephalosporanic acid is completely dissolved, and the pH of the solution is 6.8;
[0081] a-2. Add 4mol / l hydrochloric acid and an organic solvent, the organic solvent is a mixed solvent with a volume ratio of ethanol and ethyl acetate of 2:1, and the volume of the added organic solvent is 7-aminocephalosporanic acid suspension 2 times of the liquid volume; when the pH value is 3.5, stop adding hydrochloric acid dropwise, and continue to add organic solvent;
[0082] a-3. After adding the organic solvent, grow the crystals for 2 hours, filter, wash, and dry in vacuum to obtain 7-aminocephalosporanic acid crystals. Its X-ray diffraction curve is as Figure 14 shown;
[0...
Embodiment 2
[0100] Example 2. Preparation of 3-decarbamyl cefuroxime acid
[0101]
[0102] a. Measure 32ml of methanol and 18ml of NaOH solution (15%), stir and cool down to -16~-20°C, maintain at this temperature, add the condensation solution and 6ml of water at 2°C dropwise; after adding, keep the temperature of the solution Stir for 15 to 20 minutes at -16 to -20°C;
[0103] b. Add 5.4ml of glacial acetic acid dropwise, and heat up the water bath. When the temperature of the solution reaches 5-10°C, add 0.10g each of hydrosulfite and ethylenediaminetetraacetic acid (EDTA), continue stirring, and then add 75ml of dichloromethane. At this time, The temperature will rise, and the pH value of the solution will be between 5 and 6;
[0104] c. Add 26ml of HCl solution (16%) dropwise, adjust the pH value of the solution to 2.0, then continue to stir for 30 minutes, cool down to 5°C, and keep stirring for 30 minutes;
[0105] d. Filtrate, wash 3 times with 2°C water, 2 times with dichloro...
Embodiment 3
[0106] Embodiment 3. The preparation of cefuroxime acid
[0107]
[0108] a. Add 85ml of anhydrous tetrahydrofuran into the reaction bottle, cool down to 1-4°C, add decarbamoylcefuroxine under the condition of nitrogen filling, and stir until completely dissolved;
[0109] b. Cool down the solution to -70°C, and add 5.2ml of chlorosulfonyl isocyanate (CSI) dropwise while continuing to cool down; after the addition, the solution temperature should not exceed -50~-45°C;
[0110] c. Stir rapidly at -50~-45℃ for 30 minutes, add 18ml of 2℃ water dropwise, keep the solution temperature at 0~5℃, and continue stirring for 10 minutes;
[0111] d. Add NaHCO to the reaction flask 3 solution (NaHCO 3 18.00g + pure water 140ml, warm up to 35-40°C and stir to dissolve), adjust the pH value of the solution between 6.5-7.0;
[0112] e. After stirring for 10 minutes, add 72ml of ethyl acetate, stir well, and the pH of the aqueous phase is 6;
[0113] f. The temperature of the water bath...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com